Abstract
To understand quality of coking coal possessing different particle sizes, coal samples from Gao Yang (GY) and Shui Yu (SY) mines were systematically studied. The samples had wide particle size variations with six distinct ranges. The main emphasis was placed on analyzing sulfur and coke contents as function of particle sizes. X-ray photoelectron spectroscopy (XPS) was used to analyze sulfur content as well as its chemical state. Sulfur coordination and group geometry was analyzed by Fourier transform infrared spectroscopy (FTIR). Scanning electron microscopy (SEM) was applied to characterize surface morphology.
Sieving of the coal samples increased ash and sulfur contents in all fractions. Organic sulfur was mostly present as thiophene for all particle size ranges. Macromolecular structure of coal was almost the same for all samples. Changes in total sulfur content of different coal fractions were mainly caused by the variations in inorganic sulfur concentrations because organic sulfur content remained almost constant. Understanding particle size distribution in different coals is important for selecting and optimizing appropriate desulfurization methods.
Similar content being viewed by others
1 INTRODUCTION
Coal is a complex and ununiform mixture of organic and inorganic compounds [1]. Sulfur present in coal can be of organic or inorganic origin [2]. Presence of inorganic sulfur in coal is due to the sedimentary environment of the coal seam, the degree of reduction and the degree of iron ion (Fe2+ and Fe3+) enrichment around the coal seam. The major inorganic sulfur containing species in coal are pyrite, sulfates, elemental sulfur and sulfides [3].Origin of organic sulfur in coal is from plants and microbial proteins, which participated in coal formation. Organic sulfur is actually chemically incorporated into the macromolecular structure of coal [4]. However, sulfur presence is detrimental to the coking coal quality because it negatively affects properties of the resulting steel [5]. The level of coke sulfur directly affects the production of blast furnace ironmaking, and the product with sulfur content greater than 0.07% is waste. Moreover, in China, the price difference between high sulfur coal (S > 1.5%) and low sulfur coal (S < 1.0%) is very large. Therefore, to meet coking requirements and the rational use of resources, sulfur content in coal must be decreased even further.
Coal preparation removes most of the inorganic sulfur (majority of which is present as pyrite). However, High-sulfur coking coal, such as high-sulfur fat coal and main coking coal, is mostly rich in organic sulfur and difficult to remove [6]. Chemical methods of coal desulfurization, such as oxidative desulfurization, can effectively remove inorganic and organic sulfur in coal, but decrease coal quality. For a more thorough desulfurization, it is often advantageous to grind coal to certain small particle sizes to make desulfurization more efficient. To achieve the most effective desulfurization effect, the least impact on coal is a contradiction. Therefore, analysis of sulfur distributions as function of coal particle size is necessary for successful and most economical desulfurization.
Particle size is one of the most critical physical properties of coal. Coal particle size affects quality, heat transfer and chemical reaction rate during coal processing and utilization [7]. In fact, most experimental studies dedicated to coal are focused on correlation between coal particle size and its macroscopic and microscopic characteristics. A lot of studies analyzed presence and contents of organic and inorganic compounds in different coal grades using particle size fractionation. Spears et al [8] studied elemental compositions of different fractions of pulverized coal as function of their particle sizes and reported that most of the compounds concentrated in the coal fraction with particle sizes below 10 μm, while pyrite was mostly present in ~50 μm particles. Another study reported that when coal particle sizes decreased from 200 to 10 μm, ash content increased significantly [9]. The smaller the coal particles, the greater the number of free crystals or pyrite agglomerates are [10]. Particle size also significantly affects coal shape, density, surface area and pore structure. Many physical and chemical changes occurring during coal desulfurization occur on the surface and inside particle pores. Thus, coal particle sizes significantly affect desulfurization results [11–13]. Coal fineness, which is defined as percentage of material with particle sizes below 3 mm, is commonly used in coking industry to describe particle size distribution of coking coal. Coking coal fineness affects coking production, especially the yield and strength of a coke oven [14–16].
Extensive research was performed on inorganic sulfur (e.g. pyrite) accompanying various aspects of coal mining, processing and use [17, 18]. Some studies on presence of organic sulfur in coal were also published [19]. However, it is still not very clear how particle separation processes (like screening and sieving) affect sulfur distribution in fractions with different particle sizes. XPS analysis can provide quantitative data on chemical composition at the surface as well as on element chemical states (including valence and configuration) [20–22]. SEM is very useful to analyze surface morphology of coal samples and to help with distinguishing between organic and inorganic coal components [23, 24]. Surface functional groups can be studied by FTIR [25, 26]. Combination of all these instrumental tools allows for understanding of how sulfur with different contents and chemical states is distributed in coal fractions with different particle sizes.
In this work, two different types of coking coals, Gao Yang(GY) coking coal and Shui Yu(SY)fat coals, were selected. All coal samples were divided into six different fractions based on their particle sizes, after which their screening yield, morphology, surface functional groups as well as contents of ash, sulfur and coke were analyzed. All this information was then used to assess best parameters for desulfurization or coking of coal fractions with specific properties, since coal requires different desulfurization approach depending on its particle size distribution. Recommendations provided in this work can be used for efficient utilization of scarce coking coal resources.
2 EXPERIMENTAL
2.1 Techniques and Procedures
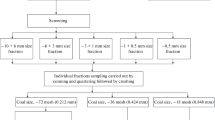
Initial materials were Gao Yang coking coal (GY) and Shui Yu fat coal (SY) with different coal ranks, which were pre-washed in a coal preparation plant. Prior to experiments, coal samples were dried at 50°C for 24 hours and then sieved to 3 mm or less. 2 kg of coal samples with particles below 3 mm were further sieved to obtain six different fractions with particle sizes in the 3-1, 1-0.45, 0.45-0.125, 0.125-0.098, 0.098-0.075 and 0.075-0 mm ranges. Fractions from each coal type (GY or SY) were labeled as I, II, III, IV, V and VI, respectively. Each fraction was weighed, after which its yield was calculated. All fractions were characterized using FTIR, XPS and SEM. Chemical state and content of sulfur in different samples as well as surface functional groups and morphology were also analyzed. Summary of experiments performed for all coal samples is given in Fig. 1. Values of the yield, coke and proximate analysis of all fractions are shown in Table 1.
2.2 Instrumental Analyses
X-ray photoelectron spectroscopy (XPS) was performed using AXISULTRADLD instrument from Kratos Analytical (UK) using Al Kα radiation (with hν = 1486.6 eV) as an X-ray source, the detection error is 0.1%. Sample analysis area was ~700 × 300 μm. Other measurement parameters were 150 W working power, 160 eV full scanning transmissivity, 40 eV narrow scan transmission energy, 10–8 Pa vacuum and 0.05 eV step size, respectively. Binding energy correction to account for sample charging was performed using C ls peak energy equal to 284.6 eV. Peaks were fitted and processed using Casa XPS software.
Microstructure and morphology of the samples were characterized by scanning electron microscopy (SEM) performed using JSM-5900lv (JEOL, Japan) at 30 kV electron beam acceleration voltage.
FTIR analysis was performed using EQUINOX 55 FTIR spectrometer (Bruker, Germany). Sample preparation involved mixing coal and KBr together (at 1:100 ratio), thoroughly grinding them and then pressing into pellets until they became clear. The pellets were then placed into a FTIR sample chamberand scanned 32 times at room temperature with 4.0 cm–1 spectral resolution in the 600–4000 cm–l scanning range.
3 RESULTS AND DISCUSSION
3.1 Coal Properties Change with the Sieve Fraction
Yields of all six fractions as function of the average particle size are shown in Fig. 2. Yields of GY and SY coals were very close for fractions with the same particle sizes. As average particle size of the coal sample decreased, the yield decreased. Particle size distribution curves exhibited a W-like shape with the lowest levels positioned at 1-0.45 and 0.098–0.074 mm. Majority of the particles for both coals, which accounted for ~50%, mainly concentrated in the 3-1 mm range. SY coal yield was slightly higher than that of the GY coal. Second largest group of particles, accounted for ~30%, concentrated in the 1-0.45 and 0.45–0.125 mm fractions. Yield for GY particles was slightly higher than for SY particles in the 0.45–0.125 mm fraction.
Results of the proximate analysis for each fraction are shown in Fig. 3. After grinding, moisture content of the coarse fractions from the SY coal was higher than of the fine ones. GY coal showed a reversed trend (see Fig. 3a,b). These phenomena are related to how moisture is bonded in specific coal type and to coal coalification degree. Moisture content gradually increased as particle size decreased mostly because of the corresponding surface area increase.
Ash mainly concentrated in the fine fraction (<0.125 mm, see Fig. 3d–f). Most of the ash is typically washed away during cleaning process at the coal processing factory. The remains are mostly finely dispersed clay minerals, which are the main ingredients of the ash in our samples. In general, these minerals are separated from the organic matter. Separated ash is finer than the organic matter, thus, more ash accumulates in the fine fraction obtained during sieving. Ash content varies greatly for various fractions of different coal rank. Ash content in the samples obtained from the GY and SY coals first decreased as particle size decreased to 1–0.45 mm but then increased again when particles were 0.45 mm and below.
Volatile component content is an important indicator reflecting bonding characteristics of coal particles. In our samples, it decreased as particle size increased. Volatiles content in SY coal was higher than in the GY coal.
Coke residues characteristics are shown in Fig. 4. GY coal coke samples formed flat blocks with poorly distinguishable coal particle boundaries. Sample showed silver-white metallic luster on the surface. Coloration was more pronounced on the lower surface of the samples. GY coal coke belongs to type 5. GY coke properties did not change much with particle size because of very small difference in composition (which typically affects bonds in coal) between the fractions. Adhesion of SY coal was stronger than of GY coal. Surface of the SY-II, SY-III and SY-IVcoal coke sample showed silver-white metallic luster, which expanded but not beyond 15 cm. Thus, SY-II, SY-III and SY-IVcoke belongs to type 6. Other fractions of SY coke also showed silver-white metallic coloration on both upper and lower surfaces of the pucks. Some gloss can be observed as well. Small bubbles are visible as well (see Fig. 4), which also confirms that SY coal belongs to type 6. SY and GY coals belong to different ranks because of their bulk compositions as well as compositions of the various fractions. The most differences were observed for the coarse-grained fractions because they contained more inert groups. Fine coal fractions typically contain more minerals; therefore, coal caking index of the fractions with particle sizes ranging from 0.45 to 1 mm was higher than of the coarse fractions.
Results of proximate analysis described above confirmed that particle size, a macroscopic parameter, is very important and needs to be always taken into account during coal processing because it affects microscopic coal properties.
3.2 Distribution of Sulfur in Coal Samples with Different Particle Size
3.2.1. Effect of coal particle size on total sulfur. Sulfur content in both coals (GY and SY) increased as particle size decreased (see Fig. 5). Sulfur content in SY coal gradually and almost linearly increased at III-V fraction (~3–0.45 mm), after which the increasing trend slowed down, and a slight decrease at <0.074 mm fraction was observed. Total sulfur content was started to rise rapidly from the III-samples (containing particles in the 0.125–0.45 mm range), and a distinct inflection point formed at the V-fraction that contained particles in the 0.074–0.098 mm range.
Total sulfur and ash contents in coal with initial high sulfur content followed the same trend: both increased as particle size decreased (Fig. 6). Sulfur in coal is typically associated with minerals (e.g. pyrite, sulfate).Overall high ash and sulfur content in fine coal fractions is because of the mineral.
Coal is a heterogeneous material [27, 28] with variable hardness [29]. During grinding, weaker coal components, like clay minerals, tend to concentrate in fine fractions [30, 31], while large-grain coal accumulates organic matter. Thus, particle size is a key factor affecting ash and sulfur distribution. During mechanical processing of coal, reduction of particle size and separation of minerals are closely related. As particle sizes of the mineral inclusions decrease, minerals gradually separate from organic matter in coal. Inorganic sulfur-containing minerals are enriched in fine fraction. Most of the inorganic sulfur in coal originates from pyrite and sulfate [32], which accumulates in coal as small and large inclusions. During sieving, particle size decreases, which contributes to higher content of inorganic sulfur inclusions. Therefore, as the particle size decreases, the total sulfur content decreases.
3.2.2. Organic sulfur content as function of particle size. We used S 2P3/2 and S 2P1/2 XPS peaks obtained for GY and SY coal samples to analyze organic sulfur content and chemical configuration. Ratio between areas of these two peaks was ~2:1, while LG% and the FWHM (full width at half maxima) value were the same. Deconvolution of S 2P3/2 peak resulted in several peaks, which can be attributed to mercaptan or thioether (at ~162.1–163.6 eV) [33–35],thiophene (at ~164.0–164.4 eV) and sulfoxide (at ~165.0–166.0 eV) [36].
Majority of sulfur in our coal samples was of organic origin (see Fig. 7a,b). It accounted for ~80% of the total sulfur content. According to XPS data, organic sulfur is mainly present in thiophene configuration, followed by sulfoxide (sulfone) and then by mercaptan and thioether.
Particle size also affects valence and configuration of organic sulfur in coal. Contents of sulfur in both GY and SY coals in mercaptan, thioether and thiophene configurations increased as grain size from I to VI (particle size decreased), while content of high valence sulfur from sulfone and sulfate gradually decreased. Proportion of organic sulfur in different grades of coal changed, but the form of organic sulfur did not.
Figure 8 shows variations of sulfur in different chemical configurations as function of particle size. Both GY and SY coals mainly contained organic sulfur in the form of thiophene. As GY coal particle size decreased, sulfone and sulfate contents first decreased but then increased. No meaningful tendencies were observed for SY coal fractions.
3.3 Morphologies of Different Coal Fractions
SEM analysis showed that sieving of GY and SY coal samples resulted in overall uniform particle size distribution in each fraction (see Fig. 9). Different particle sizes of each fraction can be clearly distinguished from the macroscopic scan image in the upper right corner. Surface of SY coal dense particles are smooth without pores. Surface of GY coal particles exhibited fine pore structure. Overall, particles are more loosely distributed in GY coal because of low level of coalification and, as a result, high mineral content.
Color of the minerals present in coal (which is bright white) is different from the color of the matrix (which is dark grey). Additionally, many impurities (such as clay minerals) were observed on the surface of almost all coal samples [37, 38]. Different types of clay minerals have different shapes (such as ribbons, strips, plates, sheets, etc. [39]) because of their different crystalline structures and morphologies. On a microscopical level, granular and flaky clastic particles can also attach to coal surface. In general, it was reported, that coals can contain three kinds of clay minerals: montmorillonite (in the form of irregular sheets), kaolinite (as hexagonal plates) and illite (as floc-like particles) [40].
During mechanical process of coal and during its washing, minerals get separated from the organic matter. Minerals present in coal exhibit different associations with organics [41, 42]. Fine clay minerals adhere to the surface of the coal particles and are easily removed during mechanical screening, which changes their content depending on the particle size. Inserts in upper right corners show macroscopic images of the sieved fractions.
3.4 Analysis of Functional Groups as Function of Coal Particle Size
Analysis of surface functional groups of lignite (also known as brown coal) fractions with different particle sizes showed that fingerprint region provided the most accurate data when coal particle sizes were in the 0.096–0.100 mm range [43]. Figure 10 exhibits the FT-IR spectra of the two coal samples of six different particle sizes. In the rough, the six spectral lines attributed to GY or SY coal of six different particle size are similar, revealing that the overall chemical structure of GY or SY coal was not changed, significantly after Screening.
FTIR spectra of GY coal fractions showed differences in peaks located at 468, 540 and 746 cm–1 (see Fig. 10a) with peak at 540 cm–1 almost disappearing for GY-III and GY-IV fractions. Peaks in the range of 1000 cm−1 to 400 cm−1 mainly derive from the C–H bonds in aromatics and mineral matters, and the stretching vibration of C–S in linear mercaptan and thioether C–S–C, at 475 cm−1 and 535 cm−1, respectively. The peak of GY coal is related to the minerals in the 1000–1200 cm–1 range. Peak intensity of the GY coal fractions decreased as particle sizes decreased but then increased. Similar trend was observed for the ash content in these coal fractions. Overall trends (peak position, intensity and appearance) observed for FTIR spectra of GY coal samples with different particle sizes did not change substantially, which indicates that molecular structure of the coal fractions was not modified significantly during mechanical treatment. Changes in FTIR spectra for the SY coal samples were mainly visible in the 500–1000 cm–1 region. Absorption peaks below 1000 cm–1 were because of presence of C–H in aromatics and in some inorganic minerals. Absorption peaks at 475 and 535 cm–1 were attributed to stretching vibrations of C–S and C–S–C groups. Peak at ~2930 cm–1 was attributed to absorption of stretching vibration of alkane methyl and methylene groups. Peak at 3400 cm–1 was ascribed to –OH vibrations, basically no change.
4 CONCLUSIONS
Mechanical processing and particle size separation leads to uneven distribution of minerals and organic matter in different coal fractions. GY coking coal and SY fat coal, belonging to different coal kinds and used in this study as experimental materials, showed similar trends as function of fraction particle sizes. The most affected properties were sulfur, ash and coke contents. Both contents of sulfur and ash increased as particle sizes decreased. Highest sulfur content was determined for the fine coal fractions. Thus, fine coal with high sulfur content can be discarded during desulfurization process or used other methods to remove.
We observed significant amount of clay minerals on the coal surface. Screening causes separation of these clay minerals from the coal matrix. SY coal demonstrated denser surface structure and higher volatile content than GY coal. The degree of coalification of SY fat coal is higher than of GY coking coal, which is the main reason for their different coking characteristics. Organic structure of our coal fractions did not change significantly as particle sizes decreased. Sulfur present in both GY and SY coals was mainly organic with thiophene accounting for most of it. Fractionation also affected FTIR spectra in the fingerprint region. Most absorption peaks, which underwent the most changes as particles sizes changed, originated form ash and sulfides (peak from 1000 cm−1 to 400 cm−1). Other peak positions did not show changes as particle sizes decreased, which indicates overall stability of the coal structure.
REFERENCES
Ward, C.R., Int. J. Coal Geol., 2002, vol. 50, no. 1, p.135. https://doi.org/10.1016/S0166-5162(02)00117-9
Chou, C.L., Int. J. Coal Geol., 2012, vol. 100, p. 1. https://doi.org/10.1016/j.coal.2012.05.009
Mathews, J.P. and Chaffee, A.L., Fuel, 2012, vol. 96, p. 1. https://doi.org/10.1016/j.fuel.2011.11.025
Zhong, S.T., Zhao, W., Sheng, C., Xu, W., Zong, Z.M., and Wei, X.Y., Energ. Fuel, 2011, vol. 25, no. 8, p. 3687. https://doi.org/10.1021/ef200844a
Ulanovskii, M.L. and Miroshnichenko, D.V., Coke Chem., 2008, vol. 51, no. 2, p. 51. https://doi.org/10.3103/s1068364x08020038
Li, A., Li, P., and Chen, S.M., J. China Coal Soc., 2007, vol. 32, no.6, p. 639.
Song, J., Hua, Z., Guo, Z., Amp, X.I., and Co, S., Fuel Chem. Process., 2015, vol. 46, no. 3 p. 7.
Spears, D.A. and Booth, C.A., Fuel, 2002, vol. 81, no. 5, p. 683. doihttps://doi.org/10.1016/s0016-2361(01)00156-9
Cloke, M., Lester, E., and Belghazi, A., Fuel, 2002, vol. 81, no. 5, p. 699. https://doi.org/10.1016/S0016-2361(01)00162-4
Zhao, H., Bai, Z., Bai, J., Guo, Z., Kong, L., and Li, W., Fuel, 2015, vol. 148, no.15, p. 145. https://doi.org/10.1016/j.fuel.2015.01.104
Ratanakandilok, S., Ngamprasertsith, S., and Prasassarakich, P., Fuel, 2001, vol. 80, no. 13, p. 1937. https://doi.org/10.1016/S0016-2361(01)00047-3
Mesroghli, S., Yperman, J., Reggers, G., Jorjani, E., and Carleer, R., Fuel, 2016, vol. 183, p. 278. https://doi.org/10.1016/j.fuel.2016.06.037
Chen, P., Zhang, X., and Xue, G., J. Wuhan Eng. Inst., 2015, vol.27, no. 2, p.4.
Xu, R., Dai, B., Wei, W., Schenk, J. and Xue, Z., Fuel Process. Technol., 2018, vol. 173, p. 11. https://doi.org/10.1016/j.fuproc.2018.01.006
Ulanovskii, M.L. and Miroshnichenko, D.V., Coke Chem., 2008, vol. 51, no. 2, p. 51. https://doi.org/10.3103/s1068364x08020038
Dmitriev, A.N., Vitkina, G.Y., and Chesnokov, Y.A., Adv. Mater. Res., 2012, vols. 602–604, p. 365. doi 10.4028/www.scientific.net/amr.602-604.365
Cai, Y., Pan, Y., Xue, J., Sun, Q., Su, G., and Xiang, L., Appl. Surf. Sci., 2009, vol. 255, no. 21, p. 8750. https://doi.org/10.1016/j.apsusc.2009.06.028
Gornostayev, S., Rkki, J.H., and Kerkkonen, O., Fuel, 2009, vol. 88, no. 10, p. 2032. https://doi.org/10.1016/j.fuel.2009.02.044
Gu, Y., Yperman, J., Reggers, G., Carleer, R., and Vandewijngaarden, J., Fuel, 2016, vol. 184, no. nov.15, p. 304. https://doi.org/10.1016/j.fuel.2016.06.085
Li, P.S., Hu, Y., Yu, W., Yue, Y.N., Xu, Q., Hu, S., Hu, N.S., and Yang, J., J. Hazard. Mater., 2009, vol. 167, no. 1–3, p. 1126. https://doi.org/10.1016/j.jhazmat.2009.01.115
Nowicki, P., Pietrzak, R. and Wachowska, H., Energ. Fuel, 2010, vol. 24, no. 2, p. 1197. https://doi.org/10.1021/ef900932g
Pietrzak, R. and Wachowska, H., Fuel Process. Technol., 2006, vol.87, no. 11, p. 1021. https://doi.org/10.1016/j.fuproc.2006.08.001
Xia, W., Xie, G., and Peng, Y., Fuel, 2016, vol. 164, p. 186. https://doi.org/10.1016/j.fuel.2015.10.009
Zhang, L., Sato, A., and Ninomiya, Y., Fuel, 2002, vol. 81, no. 11, p. 1499. https://doi.org/10.1016/S0016-2361(02)00065-0
Geng, W., Nakajima, T., Takanashi, H., and Ohki, A., Fuel, 2009, vol. 88, no. 1, p. 139. https://doi.org/10.1016/j.fuel.2008.07.027
Mishra, A.K., Chattopadhyay, D.K., Sreedhar, B., and Raju, K.V.S.N., Progr. Org. Coatings, 2006, vol. 55, no. 3, p. 231. https://doi.org/10.1016/j.porgcoat.2005.11.007
Tang, L., Chen, S., Wang, S., Tao, X., He, H., and Fan, H., Energ. Fuels, 2017, vol.31, no.12, p. 13248. https://doi.org/10.1021/acs.energyfuels.7b02112
Feng, L., Zhao, G., Zhao, Y., Zhao, M., and Tang, J., Fuel, 2017, vol.203, p. 924. https://doi.org/10.1016/j.fuel.2017.04.112
Li, J., Du, C., and Bao, J., Int. J. Mining Sci. Technol., 2010, vol. 20, no. 4, p. 611.
Yang, D., Li, J., Zheng, K., Du, C., and Liu, S., Int. J. Coal Prep. Util., 2016, no. 2, p.1. https://doi.org/10.1080/19392699.2016.1207634
Baruah, M.K. and Gogoi, P.C., Fuel, 1998, vol. 77, no. 77, p. 979. https://doi.org/10.1016/S0016-2361(97)00278-0
Marland, S., Merchant, A., and Rowson, N., Fuel, 2001, vol. 80, no. 13, p. 1839. https://doi.org/10.1016/S0016-2361(01)00050-3
Leiro, J.A., Mattila, S.S., and Laajalehto, K., Surf. Sci., 2003, vol. 547, nos. 1–2, p. 157. https://doi.org/10.1016/j.susc.2003.09.033
Grzybek, T., Pietrzak, R., and Wachowska, H., Fuel Process. Technol., 2002, vol. 77, p. 1. https://doi.org/10.1016/S0378-3820(02)00058-9
Pietrzak, R., Grzybek, T., and Wachowska, H., Fuel, 2007, vol. 86, no. 16, p. 2616. https://doi.org/10.1016/j.fuel.2007.02.025
Ma, L. L., Qin, Z.H., Zhang, L., Liu, X., and Chen, H., J. Fuel Chem. Technol., 2014, vol. 42, no. 3, p. 277.
Lin, J. S., Hendricks, R. W., Harris, L. A., and Yust, C. S., J. Appl. Crystallogr., 1978, vol. 11, no. 5, p. 621. https://doi.org/10.1107/s0021889878014065
Mishra, S. B., Langwenya, S. P., Mamba, B. B., and Balakrishnan, M., Phys. Chem. Earth Parts A/b/c, 2010, vol. 35, no. 13, p.811. https://doi.org/10.1016/j.pce.2010.07.001
Teng, J., Mastalerz, M., and Hampton, L.B., Int. J. Coal. Geol., 2017, vol. 172, p. 80. https://doi.org/10.1016/j.coal.2017.02.001
Tao, W., Yang, H., Wu, Y., Liu, Q., Lv, J., and Hai, Z., Energ. Fuel, 2012, vol. 26, no. 1, p. 990. https://doi.org/10.1021/ef201332p
Fu, B., Liu, G., Sun, M., Hower, J.C., Hu, G., and Wu, D., Fuel, 2018, vol. 228, p. 297. https://doi.org/10.1016/j.fuel.2018.04.085
Liu, Y., Gupta, R. and Sharma, A., Fuel, 2005, vol. 84, no. 10, p. 1259. https://doi.org/10.1016/j.fuel.2004.07.015
Xin, H.H., Chin. J. Spectrosc. Lab., 2012, vol. 29, no. 02, p. 690.
ACKNOWLEDGMENTS
This research was financially supported by National Nature Science Foundation of China (Grant nos. 51604144, 51774171, 5187041773). We would also like to acknowledge the financial support from the Shanxi Province Science and Technology Major Project (MJ-2016-02). We are grateful to Z. X. Jin, C. B. Deng and F. Gao for Experiment and data analysis.
Author information
Authors and Affiliations
Corresponding authors
About this article
Cite this article
Mu, X.G., Jin, Z.X., Deng, C.B. et al. Variation of Sulfur Content in Coking Coal as Function of Its Particle Size. Solid Fuel Chem. 54, 326–336 (2020). https://doi.org/10.3103/S0361521920050079
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0361521920050079