Abstract
Surface electromyography (sEMG) is a non-invasive tool that can aid physiological assessment of knee disorders towards clinical interventions. Machine Learning (ML) is widely used to classify sEMG data to help with early detection of knee disorders; however, the inherent noise and the high non-linearity of sEMG signals make pattern recognition a challenging task. This study aims to partly overcome these challenges with existing ML-based classifiers by denoising sEMG signals further via an innovative two-fold evolutionary approach. A novel Genetic Algorithm-based denoising approach is applied to sEMG data to decrease the search space for pattern-related classification. Thereafter, the proposed denoising technique is coupled with an ML-based classifier to improve the discrimination between physiological and pathophysiological knee functions from sEMG data by optimising its hyperparameters too. Thus, the novel evolutionary approach serves two purposes. Firstly, it further reduces noise in sEMG signals via a new GA-based denoising technique to concurrently maximise mutual information and minimise entropy; secondly, it also enables the optimisation of the classifier’s hyperparameters. The classification performance of the resulting hybrid algorithm was validated using sEMG data on 144 subjects (67 patients with knee disorders, 77 healthy subjects) and was found higher (ACC = 99.57%, 95% CI: 99.47–99.66; AUC = 1, 95% CI: 0.98–1) than that of similar ML algorithms and published studies. The hybrid algorithm achieved the highest classification performance by leveraging an evolutionary approach for effective denoising and hyperparameter optimisation, whilst retaining the lowest computational cost. Thus, the proposed evolutionary denoising ML-based classifier is deemed an accurate and reliable decision support system to aid the detection of knee disorders.



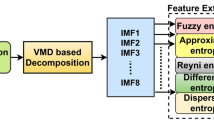
Similar content being viewed by others
References
Foroughi N, Smith R, Vanwanseele B (2009) The association of external knee adduction moment with biomechanical variables in osteoarthritis: a systematic review. Knee 16(5):303–309
Kusakunniran W, Prachasri N, Dirakbussarakom N, Yangchaem D (2017) Distinguishing ACL patients from healthy individuals using multilayer perceptron on motion patterns. In: 2017 9th international conference on knowledge and smart technology (KST), pp 1–5
Parisi L, Button K, Al-Amri H, Al-Amri M (2015) Preliminary Comparison of the Performance between the Microsoft KinectTM v2 Sensor and Vicon for Assessment of Related Functional Rehabilitation Exercises. In: Advances in Regenerative Medicine: the road to translation
Parisi L, Ravichandran N, Lanzillotta M (2020) Artificial intelligence for clinical gait diagnostics of knee osteoarthritis: an evidence-based review and analysis. TechrXiv (preprint)
Neogi T (2013) The epidemiology and impact of pain in osteoarthritis. Osteoarthr Cartil 21(9):1145–1153
Janidarmian M, Radecka K, Zilic Z (2014) Automated diagnosis of knee pathology using sensory data. In: 2014 4th international conference on wireless mobile communication and healthcare—transforming healthcare through innovations in mobile and wireless technologies (MOBIHEALTH), pp 95–98
Herrera-González M, Martínez-Hernández GA, Rodríguez-Sotelo JL, Avilés-Sánchez ÓF (2015) Knee functional state classification using surface electromyographic and goniometric signals by means of artificial neural networks. Ingeniería y Universidad 19(1):51–66
Zhang Y, Yu J, Xia C, Yang K, Cao H, Wu Q (2019) Research on GA-SVM based head-motion classification via mechanomyography feature analysis. Sensors 19(9):1986
Li Y, Gao F, Zheng X, Gan H (2017) Gait recognition using GA-SVM method based on electromyography signal. In: International conference on intelligent robotics and applications, Springer, Cham, pp 313–322
Parisi L, RaviChandran N, Manaog ML (2018) Decision support system to improve postoperative discharge: a novel multi-class classification approach. Knowl Based Syst 152:1–10
Hussain MS, Reaz MBI, Mohd-Yasin F, Ibrahimy MI (2009) Electromyography signal analysis using wavelet transform and higher order statistics to determine muscle contraction. Expert Syst 26(1):35–48
Phinyomark A, Limsakul C, Phukpattaranont P (2009) EMG denoising estimation based on adaptive wavelet thresholding for multifunction myoelectric control. In: 2009 innovative technologies in intelligent systems and industrial applications, IEEE, pp 171–176
Al Harrach M, Boudaoud S, Hassan M, Ayachi FS, Gamet D, Grosset JF, Marin F (2017) Denoising of HD-sEMG signals using canonical correlation analysis. Med Biol Eng Comput 55(3):375–388
Parisi L (2014) Exploiting kinetic and kinematic data to plot cyclograms for managing the rehabilitation process of BKAs by applying neural networks. Int J Biomed Biol Eng 8(10):664–668
De Luca CJ, Donald Gilmore L, Kuznetsov M, Roy SH (2010) Filtering the surface EMG signal: movement artifact and baseline noise contamination. J Biomech 43(8):1573–1579
Moustakidis SP, Theocharis JB, Giakas G (2010) A fuzzy decision tree based SVM classifier for assessing osteoarthritis severity using ground reaction force measurements. Med Eng Phys 32(10):1145–1160
Vincent P, Larochelle H, Lajoie I, Bengio Y, Manzagol PA (2010) Stacked denoising autoencoders: learning useful representations in a deep network with a local denoising criterion. J Mach Learn Res 11:3371–3408
Gondara L (2016) Medical image denoising using convolutional denoising autoencoders. In: 2016 IEEE 16th international conference on data mining workshops (ICDMW), IEEE, pp 241–246
Parisi L, RaviChandran N (2018) Genetic algorithms and unsupervised machine learning for predicting robotic manipulation failures for force-sensitive tasks. In: 2018 4th international conference on control, automation and robotics (ICCAR), IEEE, pp 22–25
Toledo CF, de Oliveira L, da Silva RD, Pedrini H (2013) Image denoising based on genetic algorithm. In: 2013 IEEE congress on evolutionary computation, IEEE, pp 1294–1301
de Paiva JL, Toledo CF, Pedrini H (2016) An approach based on hybrid genetic algorithm applied to image denoising problem. Appl Soft Comput 46:778–791
Jiang T, Evans DJ (2001) Image restoration by combining local genetic algorithm with adaptive pre-conditioning. Int J Comput Math 76(3):279–295
Li H, Yuan D, Ma X, Cui D, Cao L (2017) Genetic algorithm for the optimization of features and neural networks in ECG signals classification. Sci Rep 7:41011
Dioşan L, Rogozan A, Pecuchet JP (2012) Improving classification performance of support vector machine by genetically optimising kernel shape and hyper-parameters. Appl Intell 36(2):280–294
Phan AV, Le Nguyen M, Bui LT (2017) Feature weighting and SVM parameters optimization based on genetic algorithms for classification problems. Appl Intell 46(2):455–469
Sanchez OFA, Sotelo JLR, Gonzales MH, Hernandez GAM (2014) EMG dataset in lower limb data set. UCI Machine Learning Repository: 2014-02-05
Maurice M, von Tscharner V, Emery C, Nigg B (2019) Data for: Surface EMG muscle activation patterns of the lower extremities during gait in individuals with and without a knee injury history. Mendeley Data, v1. http://doi.org/10.17632/f2fv7gb577.1
Mangasarian OL, Musicant DR (2001) Lagrangian support vector machines. J Mach Learn Res 1:161–177
Parisi L, Biggs PR, Whatling GM, Holt CA (2015) A novel comparison of artificial intelligence methods for diagnosing knee osteoarthritis. In: XXV congress of the international society of biomechanics, pp 1227–1229
Parisi L, Manaog ML (2017) A minimum viable machine learning-based speech processing solution for facilitating early diagnosis of Parkinson’s disease. In: MATLAB Conference 2017
Parisi L, Manaog ML (2016) Preliminary validation of the lagrangian support vector machine learning classifier as clinical decision-making support tool to aid prediction of prognosis in patients with hepatitis. In: the 16th international conference on biomedical engineering, National University of Singapore (NUS), 2016
Parisi L, Chandran NR, Manaog ML (2018) Feature-driven machine learning to improve early diagnosis of Parkinson’s disease. Expert Syst Appl 110C:182–190
Parisi L, Chandran NR, Manaog ML (2019) A novel hybrid algorithm for aiding prediction of prognosis in patients with hepatitis. Neural Comput Appl 32(8):3839–3852
Bennasar M, Hicks Y, Setchi R (2015) Feature selection using joint mutual information maximisation. Expert Syst Appl 42(22):8520–8532
Parisi L (2014) Neural networks for distinguishing the performance of two hip joint implants on the basis of hip implant side and ground reaction force. Int J Med Heal Pharm Biomed Eng 8(10):659–663
Kohonen T (1982) Self-organized formation of topographically correct feature maps. Biol Cybern 43:59–69
Kohavi R (1995) A study of cross-validation and bootstrap for accuracy estimation and model selection. IJCAI 14(2):1137–1145
Saeb S, Lonini L, Jayaraman A, Mohr DC, Kording KP (2017) The need to approximate the use-case in clinical machine learning. Gigascience 6:1–9. https://doi.org/10.1093/gigascience/gix019
Koul A, Becchio C, Cavallo A (2018) Cross-validation approaches for replicability in psychology. Front Psychol 9:1117
Grunkemeier GL, Jin R (2001) Receiver operating characteristic curve analysis of clinical risk models. Ann Thorac Surg 72(2):323–326
Ravichandran N, Parisi L (2017) Bifurcation analysis to improve the specificity of biophysical neuronal models. In: 2017 AUT mathematical sciences symposium, vol 1(1), p 16
Abbaspour H, Mehrshad N, Razavi SM, Mesin L (2019) Artefacts removal to detect visual evoked potentials in brain computer interface systems. J Biomimet Biomater Biomed Eng 41:91–103
Favre J, Hayoz M, Erhart-Hledik JC, Andriacchi TP (2012) A neural network model to predict knee adduction moment during walking based on ground reaction force and anthropometric measurements. J Biomech 45(4):692–698
Phinyomark A, Osis ST, Hettinga BA, Kobsar D, Ferber R (2016) Gender differences in gait kinematics for patients with knee osteoarthritis. BMC Musculoskel Disorders 17(1):157
Levinger P, Lai DT, Begg RK, Webster KE, Feller JA (2009) The application of support vector machines for detecting recovery from knee replacement surgery using spatio-temporal gait parameters. Gait Posture 29(1):91–96
Begg R, Kamruzzaman J (2005) A machine learning approach for automated recognition of movement patterns using basic, kinetic and kinematic gait data. J Biomech 38(3):401–408
Parisi L, Manaog ML (2017) The importance of selecting appropriate k-fold cross-validation and training algorithms in improving postoperative discharge decision-making via artificial intelligence. In: 2017 AUT mathematical sciences symposium, vol 1(1), p 16
Acknowledgements
The authors L.P. and N.R. would like to thank the University of Auckland Rehabilitative Technologies Association (UARTA) and MedIntellego® for giving them the chance to develop this collaborative research work. This research work was carried out from January 2018 to August 2018.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Parisi, L., RaviChandran, N. Evolutionary Denoising-Based Machine Learning for Detecting Knee Disorders. Neural Process Lett 52, 2565–2581 (2020). https://doi.org/10.1007/s11063-020-10361-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11063-020-10361-1