Abstract
Background
Ataxia-telangiectasia (AT) is a rare genetic condition, caused by biallelic deleterious variants in the ATM gene, and has variable immunological abnormalities. This study aimed to examine immunologic parameters reflecting cell development, activation, proliferation, and class switch recombination (CSR) and determine their relationship to the clinical phenotype in AT patients.
Methods
In this study, 40 patients with a confirmed diagnosis of AT from the Iranian immunodeficiency registry center and 28 age-sex matched healthy controls were enrolled. We compared peripheral B and T cell subsets and T cell proliferation response to CD3/CD28 stimulation in AT patients with and without CSR defects using flow cytometry.
Results
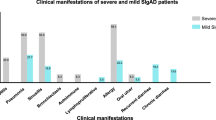
A significant decrease in naïve, transitional, switched memory, and IgM only memory B cells, along with a sharp increase in the marginal zone-like and CD21low B cells was observed in the patients. We also found CD4+ and CD8+ naïve, central memory, and terminally differentiated effector memory CD4+ (TEMRA) T cells were decreased. CD4+ and CD8+ effector memory, CD8+ TEMRA, and CD4+ regulatory T cells were significantly elevated in our patients. CD4+ T cell proliferation was markedly impaired compared to the healthy controls. Moreover, immunological investigations of 15 AT patients with CSR defect revealed a significant reduction in the marginal zone, switched memory, and more intense defects in IgM only memory B cells, CD4+ naïve and central memory T cells.
Conclusion
The present study revealed that patients with AT have a broad spectrum of cellular and humoral deficiencies. Therefore, a detailed evaluation of T and B cell subsets increases understanding of the disease in patients and the risk of infection.






Similar content being viewed by others
References
Amirifar P, Ranjouri MR, Yazdani R, Abolhassani H, Aghamohammadi A. Ataxia-telangiectasia: a review of clinical features and molecular pathology. Pediatr Allergy Immunol. 2019;30(3):277–88.
Waldmann TA. Immunological abnormalities in ataxia-telangiectasia. In: Harnden DG, Bridges BA, editors. Ataxia Telangiectasia. Sussex, John Wiley and Sons; 1982. p. 37–51.
Bobba N, Kaplan MS. Immunodeficiency and infections in ataxia-telangiectasia. Pediatrics. 2005;116(Supplement 2):568.
Moeini Shad T, Ranjouri MR, Amirifar P. ClinicalManifestations in Iranian Ataxia telangiectasia patients. Immunol Genet J. 2020;3(1):29–40.
Chessa L, Piane M, Magliozzi M, Torrente I, Savio C, Lulli P, et al. Founder effects for ATM gene mutations in Italian Ataxia telangiectasia families. Ann Hum Genet. 2009;73(5):532–9.
Birrell GW, Kneebone K, Nefedov M, Nefedova E, Jartsev M, Mitsui M, et al. ATM mutations, haplotype analysis, and immunological status of Russian patients with ataxia telangiectasia. Hum Mutat. 2005;25(6):593.
Podralska MJ, Stembalska A, Ślęzak R, Lewandowicz-Uszyńska A, Pietrucha B, Kołtan S, et al. Ten new ATM alterations in polish patients with ataxia-telangiectasia. Mol Genet Genom Med. 2014;2(6):504–11.
Kraus M, Lev A, Simon AJ, Levran I, Nissenkorn A, Levi YB, et al. Disturbed B and T cell homeostasis and neogenesis in patients with ataxia telangiectasia. J Clin Immunol. 2014;34(5):561–72.
Chao C, Yang EM, Xu Y. Rescue of defective T cell development and function in Atm−/− mice by a functional TCRαβ transgene. J Immunol. 2000;164(1):345–9.
Warnatz K, Denz A, Drager R, Braun M, Groth C, Wolff-Vorbeck G, et al. Severe deficiency of switched memory B cells (CD27+ IgM− IgD−) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002;99(5):1544–51.
Piqueras B, Lavenu-Bombled C, Galicier L, Bergeronvan der Cruyssen F, Mouthon L, Chevret S, et al. Common variable immunodeficiency patient classification based on impaired B cell memory differentiation correlates with clinical aspects. J Clin Immunol. 2003;23(5):385–400.
Wehr C, Kivioja T, Schmitt C, Ferry B, Witte T, Eren E, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111(1):77–85.
Bichuetti-Silva DC, Pereira CTM, Ferreira N, Salomão R, Brunialti MKC, Carvalho BC. Transitional B cells and CD21low in patients with ataxia-telangiectasia. World Allergy Organ J. 2015;8 Suppl 1:A63.
De Stefano A, Boldt A, Schmiedel L, Sack U, Kentouche K. Flow cytometry as an important tool in the diagnosis of immunodeficiencies demonstrated in a patient with ataxia-telangiectasia. Laboratoriumsmedizin. 2016;40(4):255–61.
Takashima T, Okamura M, Yeh T-W, Okano T, Yamashita M, Tanaka K, et al. Multicolor flow cytometry for the diagnosis of primary immunodeficiency diseases. J Clin Immunol. 2017;37(5):486–95.
Pereira C, Bichuetti-Silva D, da Mota N, Salomao R, Brunialti M, Costa-Carvalho B. B-cell subsets imbalance and reduced expression of CD40 in ataxia-telangiectasia patients. Allergol Immunopathol. 2018;46(5):438–46.
Sasihuseyinoglu AS, Yılmaz M, Bisgin A, Dogruel D, Altintas DU, Duyuler G, et al. Ataxia-telangiectasia clinical and laboratory features: single center results. Pediatr Allergy Immunol Pulmonol. 2018;31(1):9–14.
Lavin MF, Gueven N, Bottle S, Gatti RA. Current and potential therapeutic strategies for the treatment of ataxia-telangiectasia. Br Med Bull. 2007;81–82:129–47.
De Stefano A, Boldt A, Schmiedel L, Sack U, Kentouche K. Flow cytometry as an important tool in the diagnosis of immunodeficiencies demonstrated in a patient with ataxia-telangiectasia. J Lab Med. 2016;40(4):255–61.
Abolhassani H, Kiaee F, Tavakol M, Chavoshzadeh Z, Mahdaviani SA, Momen T, et al. Fourth update on the Iranian National Registry of Primary Immunodeficiencies: integration of molecular diagnosis. J Clin Immunol. 2018;38(7):816–32.
Abolhassani H, Tavakol M, Chavoshzadeh Z, Mahdaviani SA, Momen T, Yazdani R, et al. National consensus on diagnosis and management guidelines for primary immunodeficiency. Immunol Genet J. 2019;2(1):1–43.
Seidel MG, Kindle G, Gathmann B, Quinti I, Buckland M, van Montfrans J, et al. The European Society for Immunodeficiencies (ESID) registry working definitions for the clinical diagnosis of inborn errors of immunity. J Allergy Clin Immunol Pract. 2019;7:1763–70.
Amirifar P, Mozdarani H, Yazdani R, Kiaei F, Moeini Shad T, Shahkarami S, et al. Effect of class switch recombination defect on the phenotype of ataxia-telangiectasia patients. Immunol Investig. 2020;1–15.
Nissenkorn A, Levy-Shraga Y, Banet-Levi Y, Lahad A, Sarouk I, Modan-Moses D. Endocrine abnormalities in ataxia telangiectasia: findings from a national cohort. Pediatr Res. 2016;79(6):889–94.
Moin M, Aghamohammadi A, Kouhi A, Tavassoli S, Rezaei N, Ghaffari S-R, et al. Ataxia-telangiectasia in Iran: clinical and laboratory features of 104 patients. Pediatr Neurol. 2007;37(1):21–8.
Alyasin S, Esmaeilzadeh H, Ebrahimi N, Nabavizadeh SH, Nemati H. Clinical presentation of ataxia-telangiectasia. Arch Iran Med. 2019;22(12):682–686.
Jozwiak S, Janniger CK. Ataxia-telangiectasia. 2006. E-medicine from the the web. http://www.emedicine.com/derm/tpic691.htm.
Ament M. Respiratory complications of ataxia-telangiectasia. N Engl J Med. 1969;281(18):1019.
Bott L, Lebreton J, Thumerelle C, Cuvellier J, Deschildre A, Sardet A. Lung disease in ataxia-telangiectasia. Acta Paediatr. 2007;96(7):1021–4.
Nowak-Wegrzyn A, Crawford TO, Winkelstein JA, Carson KA, Lederman HM. Immunodeficiency and infections in ataxia-telangiectasia. J Pediatr. 2004;144(4):505–11.
Canny G, Roifman C, Weitzman S, Braudo M, Levison H. A pulmonary infiltrate in a child with ataxia telangiectasia. Ann Allergy. 1988;61(6):422–3, 466–8.
Driessen GJ, IJspeert H, Weemaes CM, Haraldsson Á, Trip M, Warris A, et al. Antibody deficiency in patients with ataxia telangiectasia is caused by disturbed B-and T-cell homeostasis and reduced immune repertoire diversity. J Allergy Clin Immunol. 2013;131(5):1367–75. e9.
Bredemeyer AL, Huang C-Y, Walker LM, Bassing CH, Sleckman BP. Aberrant V (D) J recombination in ataxia telangiectasia mutated-deficient lymphocytes is dependent on nonhomologous DNA end joining. J Immunol. 2008;181(4):2620–5.
Palanichamy A, Barnard J, Zheng B, Owen T, Quach T, Wei C, et al. Novel human transitional B cell populations revealed by B cell depletion therapy. J Immunol. 2009;182(10):5982–93.
Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8(1):22–33.
Isnardi I, Ng YS, Menard L, Meyers G, Saadoun D, Srdanovic I, et al. Complement receptor 2/CD21- human naive B cells contain mostly autoreactive unresponsive clones. Blood. 2010;115(24):5026–36.
Rakhmanov M, Keller B, Gutenberger S, Foerster C, Hoenig M, Driessen G, et al. Circulating CD21low B cells in common variable immunodeficiency resemble tissue homing, innate-like B cells. Proc Natl Acad Sci U S A. 2009;106(32):13451–6.
Rakhmanov M, Gutenberger S, Keller B, Schlesier M, Peter HH, Warnatz K. CD21low B cells in common variable immunodeficiency do not show defects in receptor editing, but resemble tissue-like memory B cells. Blood. 2010;116(18):3682–3.
Romberg N, Ng Y-S, Cunningham-Rundles C, Meffre E. Response: common variable immunodeficiency patients with increased CD21−/lo B cells suffer from altered receptor editing and defective central B-cell tolerance. Blood. 2011;118(22):5977–8.
Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104(12):3647–54.
Agematsu K, Nagumo H, Shinozaki K, Hokibara S, Yasui K, Terada K, et al. Absence of IgD-CD27 (+) memory B cell population in X-linked hyper-IgM syndrome. J Clin Invest. 1998;102(4):853–60.
Weller S, Faili A, Garcia C, Braun MC, Le Deist F, de Saint BG, et al. CD40-CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans. Proc Natl Acad Sci. 2001;98(3):1166–70.
Thorarinsdottir K, Camponeschi A, Gjertsson I, Mårtensson IL. CD 21−/low B cells: a snapshot of a unique B cell subset in health and disease. Scand J Immunol. 2015;82(3):254–61.
Yazdani R, Seify R, Ganjalikhani-Hakemi M, Abolhassani H, Eskandari N, Golsaz-Shirazi F, et al. Comparison of various classifications for patients with common variable immunodeficiency (CVID) using measurement of B-cell subsets. Allergol Immunopathol (Madr). 2017;45(2):183–92.
Patuzzo G, Barbieri A, Tinazzi E, Veneri D, Argentino G, Moretta F, et al. Autoimmunity and infection in common variable immunodeficiency (CVID). Autoimmun Rev. 2016;15(9):877–82.
Waldmann T, Broder S, Goldman C, Frost K, Korsmeyer S, Medici M. Disorders of B cells and helper T cells in the pathogenesis of the immunoglobulin deficiency of patients with ataxia telangiectasia. J Clin Invest. 1983;71(2):282–95.
Peterson RD, Funkhouser JD. Speculations on ataxia-telangiectasia: defective regulation of the immunoglobulin gene superfamily. Immunol Today. 1989;10(9):313–5.
Yan M, Qiang W, Liu N, Shen J, Lynn WS, Wong PK. The ataxia-telangiectasia gene product may modulate DNA turnover and control cell fate by regulating cellular redox in lymphocytes. FASEB J. 2001;15(7):1132–8.
Carney EF, Srinivasan V, Moss PA, Taylor AM. Classical ataxia telangiectasia patients have a congenitally aged immune system with high expression of CD95. J Immunol. 2012;189(1):261–8.
Garg SK, Delaney C, Toubai T, Ghosh A, Reddy P, Banerjee R, et al. Aging is associated with increased regulatory T-cell function. Aging Cell. 2014;13(3):441–8.
Jagger A, Shimojima Y, Goronzy JJ, Weyand CM. Regulatory T cells and the immune aging process: a mini-review. Gerontology. 2014;60(2):130–7.
Shiloh Y, Lederman HM. Ataxia-telangiectasia (AT): an emerging dimension of premature ageing. Ageing Res Rev. 2017;33:76–88.
Exley AR, Buckenham S, Hodges E, Hallam R, Byrd P, Last J, et al. Premature ageing of the immune system underlies immunodeficiency in ataxia telangiectasia. Clin Immunol. 2011;140(1):26–36.
Roederer M. Interpretation of cellular proliferation data: avoid the panglossian. Cytometry Part A. 2011;79(2):95–101.
Ten Brinke A, Marek-Trzonkowska N, Mansilla MJ, Turksma AW, Piekarska K, Iwaszkiewicz-Grześ D, et al. Monitoring T-cell responses in translational studies: optimization of dye-based proliferation assay for evaluation of antigen-specific responses. Front Immunol. 2017;8:1870.
Bagley J, Singh G, Iacomini J. Regulation of oxidative stress responses by ataxia-telangiectasia mutated is required for T cell proliferation. J Immunol. 2007;178(8):4757–63.
Bird JJ, Brown DR, Mullen AC, Moskowitz NH, Mahowald MA, Sider JR, et al. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9(2):229–37.
Vinuesa CG, Sze DMY, Cook MC, Toellner KM, Klaus GG, Ball J, et al. Recirculating and germinal center B cells differentiate into cells responsive to polysaccharide antigens. Eur J Immunol. 2003;33(2):297–305.
Carsetti R, Rosado MM, Donnanno S, Guazzi V, Soresina A, Meini A, et al. The loss of IgM memory B cells correlates with clinical disease in common variable immunodeficiency. J Allergy Clin Immunol. 2005;115(2):412–7.
Siebert JN, AG LH, Grillet S, Delhumeau C, Siegrist CA, Posfay-Barbe KM. Memory B cell compartment constitution and susceptibility to recurrent lower respiratory tract infections in young children. J Leukoc Biol. 2013;93(6):951–62.
Palkola NV, Pakkanen SH, Kantele JM, Pakarinen L, Puohiniemi R, Kantele A. Differences in homing potentials of Streptococcus pneumoniae–specific plasmablasts in pneumococcal pneumonia and after pneumococcal polysaccharide and pneumococcal conjugate vaccinations. J Infect Dis. 2015;212(8):1279–87.
Neill DR, Fernandes VE, Wisby L, Haynes AR, Ferreira DM, Laher A, et al. T regulatory cells control susceptibility to invasive pneumococcal pneumonia in mice. PLoS Pathog. 2012;8(4):e1002660.
Reina-San-Martin B, Chen HT, Nussenzweig A, Nussenzweig MC. ATM is required for efficient recombination between immunoglobulin switch regions. J Exp Med. 2004;200(9):1103–10.
He B, Santamaria R, Xu W, Cols M, Chen K, Puga I, et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat Immunol. 2010;11(9):836–45.
Mohammadinejad P, Abolhassani H, Aghamohammadi A, Pourhamdi S, Ghosh S, Sadeghi B, et al. Class switch recombination process in ataxia telangiectasia patients with elevated serum levels of IgM. J Immunoass Immunochem. 2015;36(1):16–26.
Malefyt W. IgA by human naive B cells is differentially IL-21-induced isotype switching to IgG and IL-21-induced isotype switching to IgG and IgA by human naive B cells is differentially regulated by IL-4 1. J Immunol Ref. 2008;181:1767–79.
Park SR, Seo GY, Choi AJ, Stavnezer J, Kim PH. Analysis of transforming growth factor-β1-induced Ig germ-line γ2b transcription and its implication for IgA isotype switching. Eur J Immunol. 2005;35(3):946–56.
Aghamohammadi A, Imai K, Moazzami K, Abolhassani H, Tabatabaeiyan M, Parvaneh N, et al. 14 Ataxia-telangiectasia in a patient presenting with hyperimmunoglobulin M syndrome. J Investig Allergol Clin Immunol. 2010;20(5):442–5.
Ghiasy S, Parvaneh L, Azizi G, Sadri G, Zaki Dizaji M, Abolhassani H, et al. The clinical significance of complete class switching defect in Ataxia telangiectasia patients. Expert Rev Clin Immunol. 2017;13(5):499–505.
Acknowledgments
The authors would like to thank Dr. Menno C. van Zelm (Monash University, Melbourne, Australia) for critical reading of the manuscript.
Funding
This research was supported by the Tehran University of Medical Sciences (grant no. 37222).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
Fig. S1. Analysis of B cell subsets in control and AT patients (i): Two examples demonstrate the flow cytometric analysis of peripheral blood samples from one control (top panels) and on the patient (down panels). Gating Strategy (ii): CD19+ B-cells are gated within the lymphocyte scatter region. Naive B-cells (C), marginal zone-like B-cells (a), IgM-only memory B-cells (a), and switched memory B-cells (b) are defined within the IgM lymphoma based on the expression of CD27 and IgD. Also, transitional B-cells (d), plasmablasts (e), and CD21low B-cells (e) are defined within the CD21 lymphogate based on the expression of CD38 and IgM. Fig. S2. Analysis of T cell subsets in control and AT patients (i): Two examples demonstrate the flow cytometric analysis of peripheral blood samples from one control (top panels) and on the patient (down panels). Gating Strategy (ii): CD4+ and CD8+ T-cells are gated within the lymphocyte scatter region. Naive, central memory, effector memory, and TEMRA T-cells (a, c) are defined within each of the CD4+ and CD8+ lymphogate based on the expression of CD45RA and CD197. Also, regulatory T-cells (b) are defined within the CD25 and FOXP3 lymphogate based on the expression of CD127. Fig. S3. Gating strategy for T CD4+ Lymphocyte proliferation. (i): Unstimulated PBMCs, labeled with the fluorescent dye CFSE, were harvested and stained with anti-human CD4 as a negative control, then analyzed by flow cytometry. (ii): A sample stimulated with anti-CD3/CD28 mAbs, labeled with the fluorescent dye CFSE, was harvested and stained with anti-human CD4, then analyzed by flow cytometry. Pseudocolor plot representing the expression of lymphocyte trafficking marker CD4 in subsequent cell divisions (right panel). (iii): We used the proliferation platform from FlowJo (v10.4.1) software in which division index, percent divided, and proliferation index are illustrated. Percent divided defines the percentage of the cells of the original sample, which is divided at least once. Proliferation index is the average number of cell divisions of responding cells and reflects the proliferative capacity of responding cells and division index, is the average number of divisions of all cells, including undivided cells. (DOCX 4299 kb)
Rights and permissions
About this article
Cite this article
Moeini Shad, T., Yousefi, B., Amirifar, P. et al. Variable Abnormalities in T and B Cell Subsets in Ataxia Telangiectasia. J Clin Immunol 41, 76–88 (2021). https://doi.org/10.1007/s10875-020-00881-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-020-00881-9