Abstract
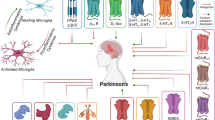
The exact etiology of Parkinson’s disease (PD) remains obscure, lacking effective diagnostic and prognostic biomarkers. In search of novel molecular factors that may contribute to PD pathogenesis, emerging evidence highlights the multifunctional role of the calcium-binding protein S100B that is widely expressed in the brain and predominantly in astrocytes. Preclinical evidence points towards the possible time-specific contributing role of S100B in the pathogenesis of neurodegenerative disorders including PD, mainly by regulating neuroinflammation and dopamine metabolism. Although existing clinical evidence presents some contradictions, estimation of S100B in the serum and cerebrospinal fluid seems to hold a great promise as a potential PD biomarker, particularly regarding the severity of motor and non-motor PD symptoms. Furthermore, given the recent development of S100B inhibitors that are able to cross the blood brain barrier, novel opportunities are arising in the research field of PD therapeutics. In this review, we provide an update on recent advances in the implication of S100B protein in the pathogenesis of PD and discuss relevant studies investigating the biomarker potential of S100B in PD, aiming to shed more light on clinical targeting approaches related to this incurable disorder.


Similar content being viewed by others
Abbreviations
- PD:
-
Parkinson’s disease
- SNpc:
-
Substantia nigra pars compacta
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- 6-OHDA:
-
6-Hydroxydopamine
- GDNF:
-
Glial-derived neurotrophic factor
- BBB:
-
Blood–brain barrier
- DAMP:
-
Damage-associated molecular pattern
- RAGE:
-
Receptor for advanced glycation end products
- HMGB1:
-
High mobility group box 1
- LIMK:
-
LIM kinase
- AP-1:
-
Activating protein-1
- COX-2:
-
Cyclooxygenase-2
- TNF-α:
-
Tumor necrosis factor- α
- CNS:
-
Central nervous system
- ROS:
-
Reactive oxygen species
- cAMP:
-
Cyclic adenosine monophosphate
- ERKs:
-
Extracellular signal-regulated kinases
- GRK2:
-
G protein-coupled receptor kinase 2
- DBS:
-
Deep brain stimulation
- SNP:
-
Single-nucleotide polymorphism
References
Radhakrishnan DM, Goyal V (2018) Parkinson’s disease: a review. Neurol India 66(Supplement):S26–S35
Angelopoulou E, Paudel YN, Piperi C (2019) miR-124 and Parkinson’s disease: a biomarker with therapeutic potential. Pharmacol Res 150:104515
Angelopoulou E, Pyrgelis ES, Piperi C (2020) Neuroprotective potential of chrysin in Parkinson’s disease: molecular mechanisms and clinical implications. Neurochem Int 132:104612
Booth HDE, Hirst WD, Wade-Martins R (2017) The role of astrocyte dysfunction in Parkinson’s disease pathogenesis. Trends Neurosci 40(6):358–370
Bahat-Stroomza M et al (2009) Induction of adult human bone marrow mesenchymal stromal cells into functional astrocyte-like cells: potential for restorative treatment in Parkinson’s disease. J Mol Neurosci 39(1–2):199–210
Chung WS et al (2013) Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504(7480):394–400
Lin LF et al (1993) GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260(5111):1130–1132
Sathe K et al (2012) S100B is increased in Parkinson’s disease and ablation protects against MPTP-induced toxicity through the RAGE and TNF-alpha pathway. Brain 135(Pt 11):3336–3347
Morales I et al (2017) Striatal astrocytes engulf dopaminergic debris in Parkinson’s disease: a study in an animal model. PLoS ONE 12(10):e0185989
Morales I et al (2016) The astrocytic response to the dopaminergic denervation of the striatum. J Neurochem 139(1):81–95
Niranjan R (2014) The role of inflammatory and oxidative stress mechanisms in the pathogenesis of Parkinson’s disease: focus on astrocytes. Mol Neurobiol 49(1):28–38
Gray MT, Woulfe JM (2015) Striatal blood-brain barrier permeability in Parkinson’s disease. J Cereb Blood Flow Metab 35(5):747–750
Sorci G et al (2010) S100B protein, a damage-associated molecular pattern protein in the brain and heart, and beyond. Cardiovasc Psychiatry Neurol 2010:656481
Donato R et al (2009) S100B’s double life: intracellular regulator and extracellular signal. Biochim Biophys Acta 1793(6):1008–1022
Liu Y, Buck DC, Neve KA (2008) Novel interaction of the dopamine D2 receptor and the Ca2+ binding protein S100B: role in D2 receptor function. Mol Pharmacol 74(2):371–378
Cristovao JS, Gomes CM (2019) S100 proteins in Alzheimer’s disease. Front Neurosci 13:463
Jiang X et al (2018) RAGE and its emerging role in the pathogenesis of Parkinson’s disease. Neurosci Lett 672:65–69
Angelopoulou E, Piperi C, Papavassiliou AG (2018) High-mobility group box 1 in Parkinson’s disease: from pathogenesis to therapeutic approaches. J Neurochem 146(3):211–218
Viana SD et al (2016a) Regulation of striatal astrocytic receptor for advanced glycation end-products variants in an early stage of experimental Parkinson’s disease. J Neurochem 138(4):598–609
Bianchi R et al (2011) S100B protein stimulates microglia migration via RAGE-dependent up-regulation of chemokine expression and release. J Biol Chem 286(9):7214–7226
Bianchi R et al (2007) S100B binding to RAGE in microglia stimulates COX-2 expression. J Leukoc Biol 81(1):108–118
Mori T, Asano T, Town T (2010) Targeting S100B in cerebral ischemia and in Alzheimer's disease. Cardiovasc Psychiatry Neurol 2010:687067
Sorci G et al (2004) S100B causes apoptosis in a myoblast cell line in a RAGE-independent manner. J Cell Physiol 199(2):274–283
Riuzzi F, Sorci G, Donato R (2006) S100B stimulates myoblast proliferation and inhibits myoblast differentiation by independently stimulating ERK1/2 and inhibiting p38 MAPK. J Cell Physiol 207(2):461–470
Riuzzi F et al (2012) S100B engages RAGE or bFGF/FGFR1 in myoblasts depending on its own concentration and myoblast density. Implications for muscle regeneration. PLoS ONE 7(1):e28700
Huttunen HJ et al (2000) Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through receptor for advanced glycation end products (RAGE) activation. J Biol Chem 275(51):40096–40105
Businaro R et al (2006) S100B protects LAN-5 neuroblastoma cells against Abeta amyloid-induced neurotoxicity via RAGE engagement at low doses but increases Abeta amyloid neurotoxicity at high doses. J Neurosci Res 83(5):897–906
Michetti F et al (2019) The S100B story: from biomarker to active factor in neural injury. J Neurochem 148(2):168–187
Rydbirk R et al (2017) Cytokine profiling in the prefrontal cortex of Parkinson’s disease and multiple system atrophy patients. Neurobiol Dis 106:269–278
Iuvone T et al (2007) Cannabinoid CB1 receptor stimulation affords neuroprotection in MPTP-induced neurotoxicity by attenuating S100B up-regulation in vitro. J Mol Med (Berl) 85(12):1379–1392
Barger SW, Van Eldik LJ (1992) S100 beta stimulates calcium fluxes in glial and neuronal cells. J Biol Chem 267(14):9689–9694
Twitchell W, Brown S, Mackie K (1997) Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol 78(1):43–50
Stampanoni Bassi M et al (2017) Cannabinoids in Parkinson’s disease. Cannabis Cannabinoid Res 2(1):21–29
Arcuri C et al (2005) S100B increases proliferation in PC12 neuronal cells and reduces their responsiveness to nerve growth factor via Akt activation. J Biol Chem 280(6):4402–4414
Bohush A, Niewiadomska G, Filipek A (2018) Role of mitogen activated protein kinase signaling in Parkinson's disease. Int J Mol Sci 19(10):2973
Al-Jarrah MD, Jamous M (2011) Effect of endurance exercise training on the expression of GFAP, S100B, and NSE in the striatum of chronic/progressive mouse model of Parkinson’s disease. NeuroRehabilitation 28(4):359–363
Gomide V, Chadi G (2005) Glial bFGF and S100 immunoreactivities increase in ascending dopamine pathways following striatal 6-OHDA-induced partial lesion of the nigrostriatal system: a sterological analysis. Int J Neurosci 115(4):537–555
Cunha MP et al (2017) MPP(+)-lesioned mice: an experimental model of motor, emotional, memory/learning, and striatal neurochemical dysfunctions. Mol Neurobiol 54(8):6356–6377
Batassini C et al (2015) Striatal injury with 6-OHDA transiently increases cerebrospinal GFAP and S100B. Neural Plast 2015:387028
Muramatsu Y et al (2003a) Cerebral alterations in a MPTP-mouse model of Parkinson’s disease—an immunocytochemical study. J Neural Transm (Vienna) 110(10):1129–1144
Muramatsu Y et al (2003b) Expression of S-100 protein is related to neuronal damage in MPTP-treated mice. Glia 42(3):307–313
Teismann P et al (2012) Receptor for advanced glycation endproducts (RAGE) deficiency protects against MPTP toxicity. Neurobiol Aging 33(10):2478–2490
Viana SD et al (2016b) Presymptomatic MPTP mice show neurotrophic S100B/mRAGE striatal levels. CNS Neurosci Ther 22(5):396–403
Hu J, Van Eldik LJ (1996) S100 beta induces apoptotic cell death in cultured astrocytes via a nitric oxide-dependent pathway. Biochim Biophys Acta 1313(3):239–245
Liu J et al (2011a) S100B transgenic mice develop features of Parkinson’s disease. Arch Med Res 42(1):1–7
Liu J et al (2017) Preliminary analysis of parkinson-like motor coordination abnormityin brain-specific hS100B transgenic mice. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 39(2):240–246
Liu JL et al (2011b) Metabonomics study of brain-specific human S100B transgenic mice by using high-performance liquid chromatography coupled with quadrupole time of flight mass spectrometry. Biol Pharm Bull 34(6):871–876
Lecours C et al (2018) Microglial implication in Parkinson’s disease: loss of beneficial physiological roles or gain of inflammatory functions? Front Cell Neurosci 12:282
Bianchi R, Giambanco I, Donato R (2010) S100B/RAGE-dependent activation of microglia via NF-kappaB and AP-1 Co-regulation of COX-2 expression by S100B, IL-1beta and TNF-alpha. Neurobiol Aging 31(4):665–677
Adami C et al (2001) S100B expression in and effects on microglia. Glia 33(2):131–142
Zhou S et al (2018) S100B promotes microglia M1 polarization and migration to aggravate cerebral ischemia. Inflamm Res 67(11–12):937–949
Schaf DV et al (2005) S100B and NSE serum levels in patients with Parkinson’s disease. Parkinsonism Relat Disord 11(1):39–43
Carvalho DZ et al (2015) Overnight S100B in Parkinson’s disease: a glimpse into sleep-related neuroinflammation. Neurosci Lett 608:57–63
Themistocleous MS et al (2017) The insertion of electrodes in the brain for electrophysiological recording or chronic stimulation is not associated with any biochemically detectable neuronal injury. Neuromodulation 20(5):424–428
Gruden MA et al (2011) Immunoprotection against toxic biomarkers is retained during Parkinson’s disease progression. J Neuroimmunol 233(1–2):221–227
Peskind ER et al (2001) Cerebrospinal fluid S100B is elevated in the earlier stages of Alzheimer’s disease. Neurochem Int 39(5–6):409–413
Wilhelm KR et al (2007) Immune reactivity towards insulin, its amyloid and protein S100B in blood sera of Parkinson’s disease patients. Eur J Neurol 14(3):327–334
Maetzler W et al (2011) Autoantibodies against amyloid and glial-derived antigens are increased in serum and cerebrospinal fluid of Lewy body-associated dementias. J Alzheimers Dis 26(1):171–179
Maetzler W et al (2014) Comparable autoantibody serum levels against amyloid- and inflammation-associated proteins in Parkinson’s disease patients and controls. PLoS ONE 9(2):e88604
Dos Santos MCT et al (2018) Evaluation of cerebrospinal fluid proteins as potential biomarkers for early stage Parkinson’s disease diagnosis. PLoS ONE 13(11):e0206536
Maarouf CL et al (2013) Quantitative appraisal of ventricular cerebrospinal fluid biomarkers in neuropathologically diagnosed Parkinson’s disease cases lacking Alzheimer’s disease pathology. Biomark Insights 8:19–28
Gmitterova K et al (2018) Cerebrospinal fluid markers analysis in the differential diagnosis of dementia with Lewy bodies and Parkinson's disease dementia. Eur Arch Psychiatry Clin Neurosci 270(4):461–470
Guo Y et al (2013) Genetic analysis of the S100B gene in Chinese patients with Parkinson disease. Neurosci Lett 555:134–136
Liu J et al (2005) SNPs and haplotypes in the S100B gene reveal association with schizophrenia. Biochem Biophys Res Commun 328(1):335–341
Roche S et al (2007) Candidate gene analysis of 21q22: support for S100B as a susceptibility gene for bipolar affective disorder with psychosis. Am J Med Genet B Neuropsychiatr Genet 144B(8):1094–1096
Fardell C et al (2018) S100B polymorphisms are associated with age of onset of Parkinson’s disease. BMC Med Genet 19(1):42
Hohoff C et al (2010) Risk variants in the S100B gene predict elevated S100B serum concentrations in healthy individuals. Am J Med Genet B Neuropsychiatr Genet 153B(1):291–297
Angelopoulou E et al (2019) The relationship between environmental factors and different Parkinson’s disease subtypes in Greece: data analysis of the Hellenic Biobank of Parkinson’s disease. Parkinsonism Relat Disord 67:105–112
Kahyaoglu I et al (2014) Umbilical CORD S100B levels in active and passive smoker women. Eur Rev Med Pharmacol Sci 18(5):723–727
Gao H et al (2018) S100B suppression alters polarization of infiltrating myeloid-derived cells in gliomas and inhibits tumor growth. Cancer Lett 439:91–100
Baudry A et al (2010) miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science 329(5998):1537–1541
Donato R et al (2013) Functions of S100 proteins. Curr Mol Med 13(1):24–57
Matsui T et al (2002) Astrocytic activation and delayed infarct expansion after permanent focal ischemia in rats. Part I: enhanced astrocytic synthesis of s-100beta in the periinfarct area precedes delayed infarct expansion. J Cereb Blood Flow Metab 22(6):711–722
Hsiao K et al (1996) Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 274(5284):99–102
Kato H et al (2004) Arundic acid, an astrocyte-modulating agent, protects dopaminergic neurons against MPTP neurotoxicity in mice. Brain Res 1030(1):66–73
Bresnick AR (2018) S100 proteins as therapeutic targets. Biophys Rev 10(6):1617–1629
Rinaldi F et al (2019) inPentasomes: An innovative nose-to-brain pentamidine delivery blunts MPTP parkinsonism in mice. J Control Release 294:17–26
Esposito E et al (2012) Neuroprotective activities of palmitoylethanolamide in an animal model of Parkinson’s disease. PLoS ONE 7(8):e41880
Goes ATR et al (2018) Protective role of chrysin on 6-hydroxydopamine-induced neurodegeneration a mouse model of Parkinson’s disease: involvement of neuroinflammation and neurotrophins. Chem Biol Interact 279:111–120
Bermejo PE, Anciones B (2009) A review of the use of zonisamide in Parkinson’s disease. Ther Adv Neurol Disord 2(5):313–317
Asanuma M et al (2010) Neuroprotective effects of zonisamide target astrocyte. Ann Neurol 67(2):239–249
Gil-Martinez AL et al (2018) Unexpected exacerbation of neuroinflammatory response after a combined therapy in old parkinsonian mice. Front Cell Neurosci 12:451
Dorszewska J et al (2014) Molecular effects of l-dopa therapy in Parkinson’s disease. Curr Genom 15(1):11–17
Esposito G et al (2014) Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-alpha activation. Gut 63(8):1300–1312
Sorci G et al (2011) The danger signal S100B integrates pathogen- and danger-sensing pathways to restrain inflammation. PLoS Pathog 7(3):e1001315
Kouli A, Horne CB, Williams-Gray CH (2019) Toll-like receptors and their therapeutic potential in Parkinson’s disease and alpha-synucleinopathies. Brain Behav Immun 81:41–51
Marxreiter F et al (2013) Glial A30P alpha-synuclein pathology segregates neurogenesis from anxiety-related behavior in conditional transgenic mice. Neurobiol Dis 59:38–51
Cirillo C et al (2011) S100B protein in the gut: the evidence for enteroglial-sustained intestinal inflammation. World J Gastroenterol 17(10):1261–1266
Santos SF et al (2019) The gut and Parkinson’s disease—a bidirectional pathway. Front Neurol 10:574
Acknowledgements
YNP would like to acknowledge Monash University Malaysia for supporting with HDR Scholarship.
Funding
This research has not received any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
EA carried out the literature review, conceptualized, and prepared the initial draft. YNP edited and contributed in the final manuscript. CP provided critical inputs, edited and contributed to the final version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Angelopoulou, E., Paudel, Y.N. & Piperi, C. Emerging role of S100B protein implication in Parkinson’s disease pathogenesis. Cell. Mol. Life Sci. 78, 1445–1453 (2021). https://doi.org/10.1007/s00018-020-03673-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-020-03673-x