Abstract
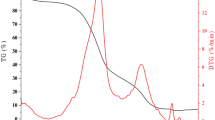
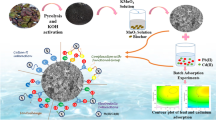
The present study elaborates the use of sagwan sawdust biochar as an efficient adsorbent for Cr(VI) abatement. Effect of experimental variables like solution pH (2–10), initial Cr(VI) concentration (30–100 mg/L), temperature (30–40 °C), adsorbent dose/Cr(VI) concentration (16.67–200), and contact time (10–80 min) was studied through batch operations. The optimization and interaction study for process parameters was done by employing response surface methodology through Box-Behnken design. The optimized condition for the maximum adsorption was pH 2.07, temperature 30.72 °C, and dose/Cr(VI) concentration 160.93. The surface characteristics of the adsorbent were evaluated via pHzpc, FTIR, BET surface area, SEM-EDS, XRD, and XPS analysis. Functional groups like O-H, C-O, C-H, and C=O were responsible for the adsorption process, and change in the oxidation state of Cr was affirmed by XPS survey. The Cr(VI) adsorption was governed by second-order kinetics and the Langmuir isotherm with Q0 of 9.62 mg/g. The thermodynamic study revealed the process to be exothermic and spontaneous. Mass transfer studies confirmed film diffusion to be favorable in the adsorption process. Adsorption occurred via electrostatic and physical attraction, reduction, and complexation. Therefore, the results suggest sagwan sawdust biochar is satisfactory and effective in removing Cr(VI) from aqueous solution.











Similar content being viewed by others
References
Sundararaju S, Manjula A, Kumaravel V, Muneeswaran T, Vennila T (2020) Biosorption of nickel ions using fungal biomass Penicillium sp. MRF1 for the treatment of nickel electroplating industrial effluent. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-00679-0
Iqbal M (2016) Vicia faba bioassay for environmental toxicity monitoring: a review. Chemosphere 144:785–802
Abbas M, Adil M, Ehtisham-ul-Haque S, Munir B, Yameen M, Ghaffar A, Abbas Shar G, Asif Tahir M, Iqbal M (2018) Vibrio fischeri bioluminescence inhibition assay for ecotoxicity assessment: a review. Sci Total Environ 626:1295–1309
Cheng C, Jia M, Cui L, Li Y, Xu L, Jin X (2020) Adsorption of Cr(VI) ion on tannic acid/grapheme oxide composite aerogel: kinetics, equilibrium, and thermodynamics studies. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-00899-4
Gardea-Torresdey JL, Tiemann KJ, Armendariz V, Bess-Oberto L, Chianelli RR, Rios J, Parsons JG, Gamez G (2000) Characterization of Cr(VI) binding and reduction to Cr(III) by the agricultural byproducts of Avena monida (Oat) biomass. J Hazard Mater B80:175–188
Vinodhini V, Das N (2009) Biowaste materials as sorbents to remove chromium (VI) from aqueous environment-a comparative study. J Agric Biol Sci 4(6):19–25
Kapur M, Mondal MK (2013) Mass transfer and related phenomena for Cr(VI) adsorption from aqueous solutions onto Mangifera indica sawdust. Chem Eng J 218:138–146
Rathore AS, Gupta GK, Kapur M, Mondal MK (2017) Study on mass transfer characteristics for Cr(VI) removal by adsorption onto residual black toner ink. Environ Prog Sustain Energy 36:1022–1029
Minas F, Chandravanshi BS, Leta S (2017) Chemical precipitation method for chromium removal and its recovery from tannery wastewater in Ethiopia. Chem Int 3(4):392–405
Chang Q, Wang G (2007) Study on the macromolecular coagulant PEX which traps heavy metals. Chem Eng Sci 62:4636–4643
Fu F, Wang Q (2011) Removal of heavy metal ions from waste waters – a review. J Environ Manag 92(3):407–418
Alkherraz AM, Ali AK, Elsherif KM (2020) Removal of Pb(II), Zn(II), Cu(II) and Cd(II) from aqueous solutions by adsorption onto olive branches activated carbon: equilibrium and thermodynamic studies. Chem Int 6(1):11–20
Akram M, Nawaz Bhatti H, Iqbal M, Noreen S, Sadaf S (2017) Biocomposite efficiency for Cr(VI) adsorption: kinetic, equilibrium and thermodynamics studies. J Environ Chem Eng 5(1):400–411
Abbas SH, Ismail IM, Mostafa TM, Sulaymon AH (2014) Biosorption of heavy metals: a review. J Chem Sci Tech 3(4):74–102
Li Q, Zhai J, Zhang W, Wang M, Zhou J (2007) Kinetic studies of adsorption of Pb(II), Cr(III) and Cu(II) from aqueous solution by sawdust and modified peanut husk. J Hazard Mater 141:163–167
Ashraf A, Bibi I, Niazi NK, Ok YS, Murtaza G, Sahid M, Kunhikrishnan A, Li D (2017) Chromium (VI) sorption efficiency of acid-activated banana peel over organo-montmorillonite in aqueous solutions. Int J Phytoremediation 19(7):605–613
Srivastava S, Agrawal SB, Mondal MK (2016) Characterization, isotherm and kinetic study of Phaseolus vulgaris husk as an innovative adsorbent for Cr(VI) removal. Korean J Chem Eng 33(2):567–575
Hyder AHMG, Begum SA, Egiebor NO (2015) Adsorption isotherm and kinetic studies of hexavalent chromium removal from aqueous solution onto bone char. J Environ Chem Eng 3:1329–1336
Liu Z, Wang G, Zhao X (2010) Removal of Cr(VI) from aqueous solution using ultrafine coal fly ash. J Wuhan Univ Technol – Mat Sci Edit 323-327
Ahamd Bhatti I, Ahmad N, Iqbal N, Zahid M, Iqbal M (2017) Chromium adsorption using waste tire and conditions optimization by response surface methodology. J Environ Chem Eng 5(3):2740–2751
Zhang HZ, Chen CR, Gray EM, Boyd SE (2017) Effect of feedstock and pyrolysis temperature on properties of biochar governing end use efficacy. Biomass Bioenergy 105:136–146
Ikenyiri PN, Abowei FMN, Ukpaka CP, Amadi SA (2019) Characterization and physicochemical properties of wood sawdust in Niger area, Nigeria. Chem Int 5(3):190–197
Wang C, Gu L, Liu X, Zhang X, Cao L, Hu X (2016) Sorption behavior of Cr(VI) on pineapple-peel-derived biochar and the influence of coexisting pyrene. Int Biodeterior Biodegradation 111:78–84
Kahraman HT, Pehlivan E (2017) Cr6+ removal using oleaster (Elaeagnus) seed and cherry (Prunus avium) stone biochar. Powder Technol 306:61–67
Gupta GK, Ram M, Bala R, Kapur M, Mondal MK (2018) Pyrolysis of chemically treated corncob for biochar production and its application in Cr(VI) removal. Environ Prog Sustain Energy 37:1606–1617
Choudhary B, Paul D (2018) Isotherms, kinetics and thermodynamics of hexavalent chromium removal using biochar. J Environ Chem Eng 6:2335–2343
Ramirez A, Ocampo R, Giraldo S, Padilla E, Flórez E, Acelas N (2020) Removal of Cr(VI) from an aqueous solution using an activated carbon obtained from teakwood sawdust: kinetics, equilibrium, and density functional theory calculations. J Environ Chem Eng 8:103702
Gupta GK, Mondal MK (2019) Bio-energy generation from Sagwan sawdust via pyrolysis: product distributions, characterizations and optimization using response surface methodology. Energy 170:423–437
APHA, AWWA, WPCF (1975) Standard methods for examination of water and wastewater, American Public Health Association, 14th ed., Washington, DC
Singh S, Chakraborty JP, Mondal MK (2019) Optimization of process parameters for torrefaction of Acacia nilotica using response surface methodology and characteristics of torrefied biomass as upgraded fuel. Energy 186:115865
Gupta GK, Gupta PK, Mondal MK (2019) Experimental process parameters optimization and in-depth product characterizations for teak sawdust pyrolysis. Waste Manag 87:499–511
Srivastava S, Agrawal SB, Mondal MK (2017) Synthesis, characterization and application of Lagerstroemia speciosa embedded magnetic nanoparticle for Cr(VI) adsorption from aqueous solution. J Environ Sci 55:283–293
Mortazavian S, Saber A, Hong J, Bae JH, Chun D, Wong N, Gerrity D, Batista J, Kim KJ, Moon J (2019) Synthesis, characterization, and kinetic study of activated carbon modified by polysulfide rubber coating for aqueous hexavalent chromium removal. J Ind Eng Chem 69:196–210
Shekari H, Sayadi MH, Rezaei MR, Allahresani A (2017) Synthesis of nickel ferrite/titanium oxide magnetic nanocomposite and its use to remove hexavalent chromium from aqueous solutions. Surf Interfaces 8:199–205
Nigam M, Rajoriya S, Singh SR, Kumar P (2019) Adsorption of Cr(VI) ion from tannery wastewater on tea waste: kinetics, equilibrium and thermodynamics studies. J Ind Eng Chem 7:103188
Khosravi R, Fazlzadehdavil M, Barikbin B, Taghizadeh AA (2014) Removal of hexavalent chromium from aqueous solution by granular and powdered Peganum Harmala. Appl Surf Sci 292:670–677
Legrouri K, Khouya E, Hannache H, El Hartti M, Ezzine M, Naslain R (2017) Activated carbon from molasses efficiency for Cr(VI), Pb(II) and Cu(II) adsorption: a mechanistic study. Chem Int 3(3):301–310
Owalude SO, Tella AC (2016) Removal of hexavalent chromium from aqueous solutions by adsorption on modified groundnut hull. Beni-Seuf Univ J Appl Sci 5:377–388
Witek-Krowiak A, Szafran RG, Modelski S (2011) Biosorption of heavy metals from aqueous solutions onto peanut shell as a low-cost biosorbent. Desalination. 265:126–134
Arvindekar AU, Laddha KS (2016) An efficient microwave assisted extraction of anthraquinones from Rheum emodi: optimization using RSM, UV and HPLC analysis and antioxidant studies. Ind Crop Prod 83:587–595
Mohammed IY, Abakr YA, Yusup S, Kazi FK (2017) Valorization of napier grass via intermediate pyrolysis: optimization using response surface methodology and pyrolysis products analysis. J Clean Prod 142:1848–1866
Dubey S, Upadhyay SN, Sharma YC (2016) Optimization of removal of Cr by -alumina nano-adsorbent using response surface methodology. Ecol Eng 97:272–283
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1367
AL-Othman ZA, Ali R, Naushad M (2012) Hexavalent chromium removal from aqueous medium by activated carbon prepared from peanut shell: Adsorption kinetics, equilibrium and thermodynamic studies. Chem Eng J 184:238–247
Tang Y, Chen L, Wei X, Yao Q, Li T (2013) Removal of lead ions from aqueous solution by the dried aquatic plant, Lemna perpusilla Torr. J Hazard Mater 244–245:603–612
Freundlich HMF (1906) Uber die adsorption in losungen. Z Phys Chem (Leipzig) 57A:385–470
Temkin MJ, Pyzhev V (1940) Recent modifications to Langmuir isotherms. Acta Physicochim. URSS 12:217–222
Wahab R, Khan F, Kaushik NK, Musarrat J, Al-Khedhairy AA (2017) Photocatalytic TMO-NMs adsorbent: temperature-time dependent Safranine degradation, sorption study validated under optimized effective equilibrium models parameter with standardized statistical analysis. Sci Rep 7:42509
Weber WJ, Morris JC (1964) Equilibria and capacities for adsorption on carbon. J Sanit Eng Div 90:79–107
Chen M, He F, Hu D, Bao C, Huang Q (2020) Broadened operating pH range for adsorption/reduction of aqueous Cr(VI) using biochar from directly treated jute (Corchorus capsularis L.) fibers by H3PO4. Chem Eng J 381(1):122739
Mohan D, Rajput S, Singh VK, Steele PH, Pittman CU Jr (2011) Modeling and evaluation of chromium remediation from water using low cost bio-char, a green adsorbent. J Hazard Mater 188:319–333
Mohan D, Singh KP, Singh VK (2005) Removal of hexavalent chromium from aqueous solution using low-cost activated carbons derived from agricultural waste materials and activated carbon fabric cloth. Ind Eng Chem Res 44:1027–1042
Acknowledgments
The authors are very obliged to the Department of Chemical Engineering and Technology and Central Instrument Facility Centre, Indian Institute of Technology, BHU, Varanasi, for imparting amenities for the completion of the research study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gupta, G.K., Mondal, M.K. Mechanism of Cr(VI) uptake onto sagwan sawdust derived biochar and statistical optimization via response surface methodology. Biomass Conv. Bioref. 13, 709–725 (2023). https://doi.org/10.1007/s13399-020-01082-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01082-5