Abstract
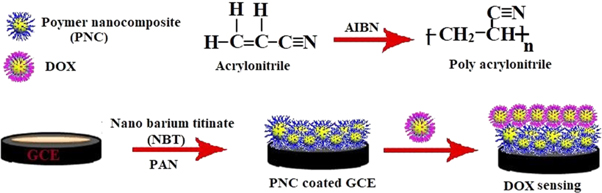
A microwave (MW) assisted process was evolved to synthesize a series of polymer nanocomposites (PNCs) through 2,2-azobisisobutyronitrile (AIBN) initiated free radical in situ polymerization of acrylonitrile (AN) in presence of nanobariumtitanate (NBT). The reaction conditions were optimized and microwave power ranging 25 to 100 W over 10 min was found to be most suited for the synthesis of PNCs. Synthesis of PNCs has been ascertained through UV–vis, FTIR spectroscopy and microstructure were investigated through XRD and AFM. TG-DTA-DTG proclaims that PNCs acquire lower moisture content and higher heat resistance as compared to polyacrylonitrile (PAN). The synthesized PNCs have been applied as sensing material to develop electrochemical probe for detection of doxorubicin (DOX). The presence of DOX (0.01%, w/v) in phosphate buffer at pH 7.4 has shown a remarkable increase in the peak current at PNCs modified glassy carbon electrode (GCE). Cyclic voltammetric (CV) studies proof good acceptance of nanocomposites as sensing material for anti cancerous drug DOX.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
DOX is an anthracycline cytostatic drug originated by streptomyces peucetius varieta caesius [1, 2]. It has been widely used in the treatment of various categories of cancers such as osteogenic sarcoma, gastric, lung, soft breast, liver, thyroid and lymphoblastic leukemia [3]. However, common side effects include bone marrow suppression, hair loss, mouth inflammation and allergic reaction [4]. Development of selective and sensitive electrochemical methods for monitoring DOX is of great clinical significance to assess the therapeutic efficiency [5, 6].
The technological significance of MW assisted techniques has been recognized as an inexpensive and green alternative for synthesis and processing of a variety of polymer based nanocomposites. It also facilitates reduction of chemical waste and reaction times [7]. In recent years the application of MW has opened up many opportunities to improve preparation of polymer based material. MW synthesis and processing of polymers and related materials can be controlled more accurately than in conventional methods [8]. It offers several advantages over conventional heating methods, which includes unique microstructure and properties, improved product yield, energy savings, faster processing time, reduction in manufacturing cost and more consistent product quality [9]. It utilizes ability of mobile electric charges present in liquid or conducting ions in solid to transform electromagnetic energy into heat [10]. However the actual implementation of this technology towards development of the novel class of nanomaterials has been lacking and the benefits have gone largely unrealized.
Although MW assisted synthesis and processing of polymers nanocomposites has been explored [11], but no attempts were made to synthesize the PNCs derived from PAN and NBT. In the present investigation, efforts have been made to synthesize the proposed PNCs through optimized loading of AIBN initiated in situ polymerization of AN in presence of NBT at different MW power conditions with various time frame were explored.
The synthesized PNCs were characterized through their spectra, microscopy and thermal analysis [12]. The electrochemical sensing behavior of PNCs towards a commercially available anti-tumor drug DOX has been investigated through CV at PNCs modified glassy carbon electrode.
2. Experimental details
2.1. Materials
AN, NBT and AIBN were purchased from SD Fine Chemicals, India. DOX of purity >99% was obtained from Innova Life Sciences Pvt. Ltd India and was used without further purification. Other chemicals and solvents (purity >98%) were indigenously procured of analytical reagent grade and used without further purification. De-ionized water was obtained from a PURE ROUP 30 water purification system was used in this experiment.
2.2. Synthesis of PAN and PNCs
A series of PNCs was prepared through AIBN initiated polymerization of AN monomer in presence of NBT at optimized MW power and time settings. Prior to polymerization reactions, the inhibitor content of AN monomer has been removed through extracting into NaOH (10%, w/w), followed by distillation under reduced pressure and remaining fraction (λmax: 221.0, density: 0.806 g dm−3) was collected to used. For the MW synthesis of PAN a glass vial (10 ml) was charged with AN (1.0 ml; 1.52 × 10–2 mol) and AIBN (50 mg, 3.05 × 10−4 mol). The contents were subjected to MW irradiation at 25–50 W power over 10 min, where PAN has been isolated as an amorphous white solid. All PNCs were synthesized through AIBN initiated free radical in situ polymerization of AN in presence of NBT. For this purpose AN (1.0 ml; 1.52 × 10−2 mol), NBT (8.0 mg, 0.35 × 10−4 mol) and AIBN (50 mg, 3.05 × 10−4 mol) were taken in a glass vial and the content were subjected to MW at power ranging 25–50 W over 10 min. The PNCs were isolated as crystalline materials, that was further proved by microstructure analysis (scheme
Scheme 1. Modification of GCE for electrochemical sensing of DOX.
Download figure:
Standard image High-resolution image2.3. Development of modified GCE electrode
GCE were modified by the depositing PAN and PNCs suspension respectively over the top surface of bare GCE (2 mm diameter). Prior to deposition of coating over GCE, it was polished with fine alumina slurry on soft diamond pad. Polished GCE was cleaned by ultrasonication in acetone/water for 5 min followed by air drying. A homogenous solution of coating materials were prepared by dissolving PNCs and PAN in tetrahydrofuran (1 mg ml−1) at 50 ± 1 °C for 15–20 min, followed by sonication (30 min). GCE was coated by applying 125 μl of prepared suspension with micropipette, followed by drying at 50 ± 1 °C, 400/mmHg for 1 h.
2.4. Instrumentations
UV–vis spectra were recorded on a Genesis 10 Thermo Spectronic spectrophotometer in NMP.
FT-IR spectra were recorded over Thermo Nicolet 6700 Spectrophotometer in KBr.
XRD spectra was recorded at 25 °C over a Rigaku Geiger flex diffractometer using Cu-Kα radiation (λ = 0.154056 nm). The XRD data were measured at room temperature over scattering angle 5°–90° at step of 0.019° using CuK radiation with graphite monochromator.
AFM images of the samples were recorded over borosilicate glass substrate using INTEGRA Prima atomic force microscope under tapping mode through ultra sharp Si cantilevers (force constant 48 N m−1).
TG-DTA-DTG was performed with an EXSTAR TG/DTA 6300 system from room temperature to 800 °C in air at a scan rate of 10 °C min−1 in presence of alumina as reference.
The CV experiments were conducted over IVIUM Potentiostat-Galvanostat using a three electrode electrochemical cell in phosphate buffer (15 ml) comprising DOX (0.01%, w/v). The cell was consisting of Ag/AgCl reference electrode, Pt counter electrode and PNCs modified GCE.
3. Results and discussions
The formation of synthesized PNCs has been ascertained through UV–vis, FT-IR, XRD spectra, AFM and simultaneous TD-DTA-DTG [13]. The application of PNCs as an electrochemical sensoring material for DOX has been explored in PBS at pH 7.4 through CV [14].
3.1. UV–vis studies
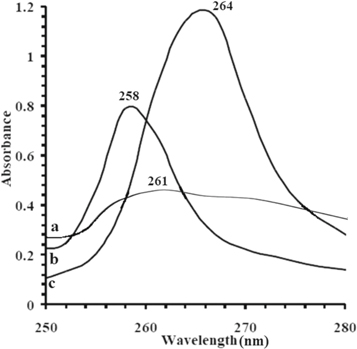
In UV–vis spectra (figure 1) NBT and PAN shows absorption at 261 and 258 nm respectively. PNCs shows absorption at 264 nm, such red shift in the absorption may be assigned to the loss of the unsaturation of the vinyl functionality as a result of polymerization reaction for formation of PNCs.
Figure 1. UV spectra of (a) NBT (b) PAN (c) PNCs respectively.
Download figure:
Standard image High-resolution image3.2. FT-IR spectroscopy
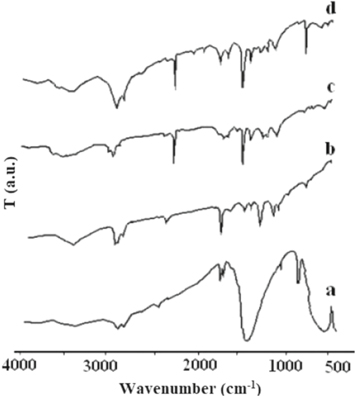
Figure 2 shows comparative FT-IR spectra (cm−1) of NBT, AN, PAN and synthesized PNCs respectively. Figure 2(a) shows FT-IR spectrum of NBT indicating characteristic wave number at 3422.5 (νTiOH), 1430.9 (δO–H), and 555.2 to 686.9 (TiO octahedral). AN shows characteristic wave numbers at 2957.3 (νas C–H), 2865.2 (νsyC–H), 2361.6 (νcyano), 1728.4 (νC=C), 1458.9 (ιC–H), 1379.8 (σC–H), 747.8 (ρC–H) (figure 2(b)).
Figure 2. FT-IR Spectra of (a) NBT (b) AN (c) PAN (d) PNCs.
Download figure:
Standard image High-resolution imagePAN shows characteristic wave numbers at 2921.6 (νas C–H), 2850.0 (νsyC–H), 2244.2 (νcyano), 1621.7 (νC=N), 1454.5 (ιC–H), 1366.6 (σC–H), 708.6 (ρC-H) (figure 2(c)). IR spectra of PNCs reflects characteristic absorption bands 2921.6 (νasC–H), 2850.0 (νsyC–H), 2244.1 (νcyano), 1457.4 (ιC–H), 1365.2 (σC–H), 721.7 (ρC–H) (figure 2(d)). It is reflected from the IR spectra that with addition of NBT in PAN, there is significant shift in (ρC–H) and a new peak appears at around 680 cm−1 due to TiO binding with PAN in the formation of PNCs [11, 12].
3.3. Microstructure analysis
The microstructure of the PNCs has been estimated, through XRD and AFM. Figure 3 shows XRD spectra of NBT and PNCs. NBT shows sharp diffraction pattern indicating nanocrystalline structure (figure 3). The XRD reveals peak characteristics of NBT at 2θ = 2.8, 2.1, and 2.0 with hkl values (101), (110), (111), (200), (002). The peak indexed to (101), (110), (200), (002), (210), (201), (102), (211), (202), (220), (212), (221), (103), (201), (310), (113), (311) and (222) planes, corresponding to crystal structure of NBT structure with tetragonal phase [15]. Calculations based on Debye–Scherrer equation indicate the existence of NBT into crystallite size 54 nm.
Figure 3. XRD spectra of (a) NBT (b) PNCs.
Download figure:
Standard image High-resolution imageIn PNCs XRD peaks were observed between (100) and (111) and BaTiO3 phase were also observed. The corresponding PNCs have shown some extent of amorphous zone in crystalline structure of PNCs with shift of 2θ to lower value indicating the exfoliation of the nanocrystalline phase of NBT into PAN matrix, that has been supported through XRD data.
3.4. AFM analysis
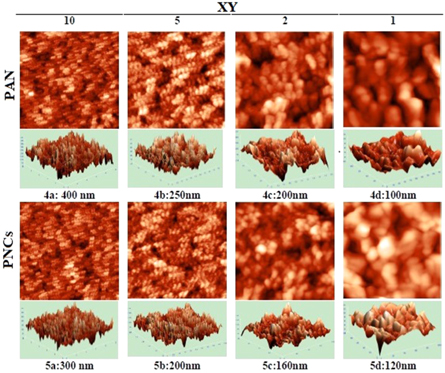
The AFM images of PAN and a representative PNCs synthesized at 25 W are shown in figure 4. The images has been scanned at various indenter heights (Z) ranging 400 to 100 nm. PAN shows slight changes as compared to PNCs in the grain morphology, with simultaneous reduction in the average and RMS roughness at all the XY scales. The microscopy data suggests that the presence of NBT has reduced the roughness of PAN matrix in the respective nanocomposites. Such reduction in the roughness of the PNCs over PAN may be attributed to the exfoliation of NBT into PAN matrix that has been supported through XRD data.
Figure 4. Comparative AFM of PAN and PNCs at different resolutions.
Download figure:
Standard image High-resolution image3.5. Thermal analysis
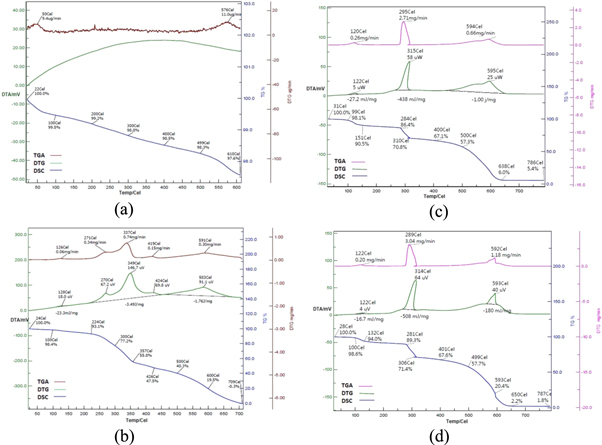
The thermal stability of NBT, PAN and PNCs have been studied through simultaneous TG-DTA-DTG (figures 5(a)–(d)). The thermal data is expressed as TG (°C) onset, TG (°C) endset, DTA (°C) and DTG (°C) peak. The DTA signals were expressed as mV and DTG showed the rate of degradation in sample and expressed as μg °C−1. Heat of fusion data was revealed through DTA and expressed in V.s mg−1.
Figure 5. (a): TG-DTG-DTA of NBT (25 W) (b): TG-DTG-DTA of PAN (25 W) (c): TG-DTG-DTA of PNCs (25 W) (d): TG-DTG-DTA of PNCs (50 W).
Download figure:
Standard image High-resolution imageThe dispersion of NBT into PAN matrix through insitu polymerization at MW power 25 W has afforded PNCs with moisture content 1.9% corresponding to WL% at 99 °C (figure 5(c)). Such release of moisture from starting material [I] was associated with a DTA exotherm at 122 °C (5 μW) with a heat of decomposition −27.2 mJ mg−1 and a DTG profile at 120 °C with rate of decomposition 0.26 mg min−1. The first step thermal decomposition [Io] of PNCs has started at 284 °C with WL% 13.6, which was further supported by a remarkable DTA exotherm at 315 °C (58 μW) with the associated heat of decomposition −438 mJ mg−1 and a DTG peak at 295 °C with rate of decomposition 2.71 mg min−1. The final step thermal decomposition [If] has been reported at 500 °C with WL% 42.7, with the corresponding DTA exotherm at 595 °C (25 μW) with heat of decomposition −1000 mJ mg−1. The corresponding DTG peak has been reported at 594 °C with rate of decomposition 0.66 mg min−1. The thermal decomposition of PNCs synthesized at MW power 25 W has been completed at 786 °C leaving the char residue (%) 5.4.
The dispersion of NBT into PAN matrix at MW power 50 W has afforded PNCs with moisture content (%) 1.4 at 100 °C (figure 5(d)). Such release of moisture was associated with a DTA exotherm at 122 °C (4 μV) with heat of decomposition −16.7 mJ mg−1 and a DTG profile at 122 °C with rate of decomposition 0.20 mg min−1. TGA data revealed that [Io] of PNCs has been started at 281 °C with WL% 10.7. This was further supported by a DTA exotherm at 314 °C (64μV) with heat of decomposition −508 mJ mg−1 and a DTG peak at 289 °C with rate of decomposition 3.04 mg min−1. [If] has been reported at 499 °C with WL% 42.3, with the corresponding DTA exotherm at 593 °C (40 μV) with heat of decomposition 180 mJ mg−1. The corresponding DTG peak has been reported at 592 °C with rate of decomposition 1.18 mg min−1. The thermal decomposition of PNCs synthesized at MW power 50 W has been terminated at 787 °C leaving the char residue (%) 1.8. The present TGA data showed higher stability of developed PNCs over NBT and PAN, which may be due to the regioregular structure of PNCs. It is also evident from thermal studies that PNCs synthesized at MW power 25 W has been found more thermally stable over PNCs synthesized MW power 50 W.
3.6. Electrochemical sensing of DOX
In the present study, the CV electrochemical studies were carried out for DOX monitoring at bare, PAN and PNCs coated GCE. Remarkable variations in the electrochemical behavior of DOX have been observed at bare, PAN and PNCs systems (figure 6(a)). The analysis of the relative current response reveals highest sensitivity of DOX at PNCs modified GCE system.
Figure 6. (a) Variation in current response with time for DOX on Bare(Black), PNCs(Green) and PAN(Red) modified GCE. (b) Cyclic Voltammograms of DOX on bare (Black), PNCs(Green) and PAN(Red) modified GCE.
Download figure:
Standard image High-resolution imageFigure 6(b), showed CV studies of DOX at bare, PAN and PNCs modified GCE with characteristic peak current reduction, that corresponding to -Ered at 0.842 V with peak current −2.52 × 10–5 A. It was found that with the increase in numbers of CV scans there is enhancement in peak current and a remarkable reduction and oxidation peaks have been rendered by PAN and PNCs modified GCE. Quantitative estimation of DOX was performed by varying the concentration of DOX in phosphate buffer at pH 7.4, and best CV response was obtained at 0.01%, w/v, at PNCs coated GCE. These results show electroactivity of PNCs due to the polymer characteristics including large specific surface area, excellent electrical conductivity, redox electroactivity and more electroactive interaction sites that could serve as a good platform for charge exchange and electron transfer between the DOX and the modified electrode surface.
4. Conclusions
In the proposed work a highly efficient approach to develop PNCs from PAN and NBT under MW irradiation have been reported. With the aid of MW irradiation, the polymerization rate and the equilibrium conversion were enhanced. Developed PNCs can be used as surface modifier at GCE for the development of DOX sensitive sensor. Microscopy data suggests that the presence of NBT has reduced the roughness of PAN matrix in PNCs due to exfoliation of NBT into PAN matrix that has also been supported through XRD data. Simultaneous TG-DTA-DTG data indicates higher thermal stability of PNCs over NBT and PAN. CV studies indicate electrochemical sensing behavior of PNCs coated GCE towards DOX, that proves its good acceptance as a sensing material for DOX. This method has advantages of being simple, rapid, low-cost, and environmentally friendly.
Acknowledgments
The authors acknowledge the department of chemistry, G B Pant University of Agriculture & Technology, Pantnagar, India for providing all the support during the study period.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary information files).