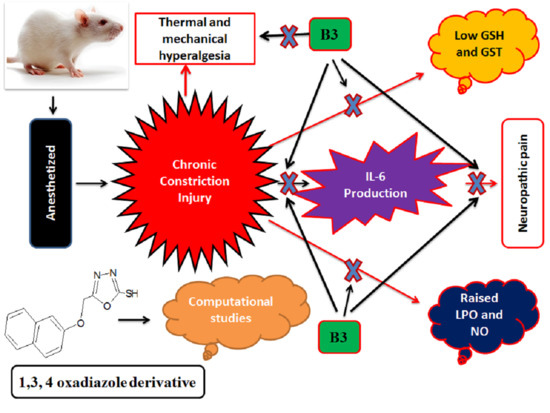
1,3,4-Oxadiazole Derivative Attenuates Chronic Constriction Injury Induced Neuropathic Pain: A Computational, Behavioral, and Molecular Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Animal Grouping and Dosing
2.4. Docking Studies
2.5. Sciatic Nerve Constriction
2.6. Behavioral Studies
2.6.1. Thermal Hyperalgesia
2.6.2. Mechanical Allodynia
2.6.3. Paw Deformation
2.7. Biochemical Investigations
2.7.1. LPO Assay
2.7.2. Nitric Oxide Assay
2.7.3. Estimation of Oxidative Stress
2.8. Hematoxylin and Eosin Staining
2.9. Immuno-Histopathological Evaluation
2.10. ELISA
2.11. Statistical Analysis
3. Results
3.1. Docking
3.2. Effect on Thermal Latency
3.3. Effect on Mechanical Allodynia
3.4. Effect on Paw Deformation
3.5. Effect on LPO and Nitric Oxide
3.6. Effect on Oxidative Stress Enzyme
3.7. Effect of Histopathological Changes (H and E and Immuno-Histochemical Changes)
3.8. Effects on Inflammatory Marker (IL-6)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaplan, H.M.; Mishra, P.; Kohn, J. The overwhelming use of rat models in nerve regeneration research may compromise designs of nerve guidance conduits for humans. J. Mater. Sci. Mater. Med. 2015, 26, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamoto, K.; Martin, D.P.; Schmelzer, J.D.; Mitsui, Y.; Low, P.A. Pro-and anti-inflammatory cytokine gene expression in rat sciatic nerve chronic constriction injury model of neuropathic pain. Exp. Neurol. 2001, 169, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Garbers, C.; Rose-John, S. Interleukin-6: From Basic Biology to Selective Blockade of Pro-Inflammatory Activities, Seminars in Immunology. Semin. Immunol. 2014, 26, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T. Interleukin-6: From basic science to medicine—40 years in immunology. Annu. Rev. Immunol. 2005, 23, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Boulanger, M.J.; Chow, D.-c.; Brevnova, E.E.; Garcia, K.C. Hexameric structure and assembly of the interleukin-6/IL-6 α-receptor/gp130 complex. Science 2003, 300, 2101–2104. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.; Wang, J.; Wang, C.; Yang, Z.; Wang, C.; Zhu, Y.; Zhang, J. Paeoniflorin and albiflorin attenuate neuropathic pain via MAPK pathway in chronic constriction injury rats. Evid. Based Complementary Altern. Med. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Faheem, M.; Khan, A.-U.; Nadeem, H.; Ali, F. Computational and pharmacological evaluation of heterocyclic 1, 3, 4-oxadiazole and pyrazoles novel derivatives for toxicity assessment, tumour inhibition, antioxidant, analgesic and anti-inflammatory actions. Farmacia 2018, 66, 909–919. [Google Scholar] [CrossRef]
- Malghani, Z.; Khan, A.-U.; Faheem, M.; Danish, M.Z.; Nadeem, H.; Ansari, S.F.; Maqbool, M. Molecular Docking, Antioxidant, Anticancer and Antileishmanial Effects of Newly Synthesized Quinoline Derivatives. Anti Cancer Agents Med. Chem. 2020, 20, 1516–1529. [Google Scholar] [CrossRef]
- Bennett, G.J.; Xie, Y.-K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Huang, C.; Hu, Z.-P.; Long, H.; Shi, Y.-S.; Han, J.-S.; Wan, Y. Attenuation of mechanical but not thermal hyperalgesia by electroacupuncture with the involvement of opioids in rat model of chronic inflammatory pain. Brain Res. Bull. 2004, 63, 99–103. [Google Scholar] [CrossRef]
- Joshi, R.P.; Negi, G.; Kumar, A.; Pawar, Y.B.; Munjal, B.; Bansal, A.K.; Sharma, S.S. SNEDDS curcumin formulation leads to enhanced protection from pain and functional deficits associated with diabetic neuropathy: An insight into its mechanism for neuroprotection. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Nakazato-Imasato, E.; Kurebayashi, Y. Pharmacological characteristics of the hind paw weight bearing difference induced by chronic constriction injury of the sciatic nerve in rats. Life Sci. 2009, 84, 622–626. [Google Scholar] [CrossRef]
- Iqbal, S.; Shah, F.A.; Naeem, K.; Nadeem, H.; Sarwar, S.; Ashraf, Z.; Imran, M.; Khan, T.; Anwar, T.; Li, S. Succinamide Derivatives Ameliorate Neuroinflammation and Oxidative Stress in Scopolamine-Induced Neurodegeneration. Biomolecules 2020, 10, 443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, K.S.; Hsieh, H.W.; Wang, S.-Y. Anti-inflammatory effect of lucidone in mice via inhibition of NF-κB/MAP kinase pathway. Int. Immunopharmacol. 2010, 10, 385–392. [Google Scholar] [CrossRef]
- Imran, M.; Al Kury, L.T.; Nadeem, H.; Shah, F.A.; Abbas, M.; Naz, S.; Khan, A.-u.; Li, S. Benzimidazole Containing Acetamide Derivatives Attenuate Neuroinflammation and Oxidative Stress in Ethanol-Induced Neurodegeneration. Biomolecules 2020, 10, 108. [Google Scholar] [CrossRef] [Green Version]
- Guedes, R.P.; Dal Bosco, L.; da Rosa Araújo, A.S.; Belló-Klein, A.; Ribeiro, M.F.M.; Partata, W.A. Sciatic nerve transection increases gluthatione antioxidant system activity and neuronal nitric oxide synthase expression in the spinal cord. Brain Res. Bull. 2009, 80, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Mohsin Alvi, A.; Tariq Al Kury, L.; Umar Ijaz, M.; Ali Shah, F.; Tariq Khan, M.; Sadiq Sheikh, A.; Nadeem, H.; Khan, A.-u.; Zeb, A.; Li, S. Post-Treatment of Synthetic Polyphenolic 1, 3, 4 Oxadiazole Compound A3, Attenuated Ischemic Stroke-Induced Neuroinflammation and Neurodegeneration. Biomolecules 2020, 10, 816. [Google Scholar] [CrossRef]
- Ali, A.; Shah, F.A.; Zeb, A.; Malik, I.; Alvi, A.M.; Alkury, L.T.; Rashid, S.; Hussain, I.; Ullah, N.; Khan, A.U. NF-κB Inhibitors Attenuate MCAO Induced Neurodegeneration and Oxidative Stress—A Reprofiling Approach. Front. Mol. Neurosci. 2020, 13, 33. [Google Scholar] [CrossRef]
- Komirishetty, P.; Areti, A.; Gogoi, R.; Sistla, R.; Kumar, A. Combination strategy of PARP inhibitor with antioxidant prevent bioenergetic deficits and inflammatory changes in CCI-induced neuropathy. Neuropharmacology 2017, 113, 137–147. [Google Scholar] [CrossRef]
- Bakare, A.O.; Owoyele, B.V. Antinociceptive and neuroprotective effects of bromelain in chronic constriction injury-induced neuropathic pain in Wistar rats. Korean J. Pain 2020, 33, 13. [Google Scholar] [CrossRef] [Green Version]
- Sakhaee, M.H.; Sayyadi, S.A.H.; Sakhaee, N.; Sadeghnia, H.R.; Hosseinzadeh, H.; Nourbakhsh, F.; Forouzanfar, F. Cedrol protects against chronic constriction injury-induced neuropathic pain through inhibiting oxidative stress and inflammation. Metab. Brain Dis. 2020, 35, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Kuhad, A.; Bhandari, R.; Prasad, S.K.; Shakya, A.; Prasad, R.S.; Sinha, S.K. Effect of ethanolic extract of Solanum virginianum Linn. on neuropathic pain using chronic constriction injury rat model and molecular docking studies. Naunyn Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1715–1728. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Chen, J.; Yu, K.; Cheng, K.; Chen, I.; Wu, P.; Wu, B. Neuroprotective and anti-inflammatory activities of atorvastatin in a rat chronic constriction injury model. Int. J. Immunopathol. Pharmacol. 2012, 25, 219–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boccella, S.; Guida, F.; De Logu, F.; De Gregorio, D.; Mazzitelli, M.; Belardo, C.; Iannotta, M.; Serra, N.; Nassini, R.; de Novellis, V. Ketones and pain: Unexplored role of hydroxyl carboxylic acid receptor type 2 in the pathophysiology of neuropathic pain. FASEB J. 2019, 33, 1062–1073. [Google Scholar] [CrossRef]
- Liang, F.; Liu, M.; Fu, X.; Zhou, X.; Chen, P.; Han, F. Dexmedetomidine attenuates neuropathic pain in chronic constriction injury by suppressing NR2B, NF-κB, and iNOS activation. Saudi Pharm. J. 2017, 25, 649–654. [Google Scholar] [CrossRef]
- Al Kury, L.T.; Zeb, A.; Abidin, Z.U.; Irshad, N.; Malik, I.; Alvi, A.M.; Khalil, A.A.K.; Ahmad, S.; Faheem, M.; Khan, A.-U. Neuroprotective effects of melatonin and celecoxib against ethanol-induced neurodegeneration: A computational and pharmacological approach. Drug Des. Dev. Ther. 2019, 13, 2715. [Google Scholar] [CrossRef] [Green Version]
- Backonja, M.-M.; Miletic, G.; Miletic, V. The effect of continuous morphine analgesia on chronic thermal hyperalgesia due to sciatic constriction injury in rats. Neurosci. Lett. 1995, 196, 61–64. [Google Scholar] [CrossRef]
- Kaur, G.; Bedi, O.; Sharma, N.; Singh, S.; Deshmukh, R.; Kumar, P. Anti-hyperalgesic and anti-nociceptive potentials of standardized grape seed proanthocyanidin extract against CCI-induced neuropathic pain in rats. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 9–17. [Google Scholar] [CrossRef]
- Chanchal, S.K.; Mahajan, U.B.; Siddharth, S.; Reddy, N.; Goyal, S.N.; Patil, P.H.; Bommanahalli, B.P.; Kundu, C.N.; Patil, C.R.; Ojha, S. In vivo and in vitro protective effects of omeprazole against neuropathic pain. Sci. Rep. 2016, 6, 30007. [Google Scholar] [CrossRef] [Green Version]
- Abbaszadeh, A.; Darabi, S.; Hasanvand, A.; Amini-Khoei, H.; Abbasnezhad, A.; Choghakhori, R.; Aaliehpour, A. Minocycline through attenuation of oxidative stress and inflammatory response reduces the neuropathic pain in a rat model of chronic constriction injury. Iran. J. Basic Med Sci. 2018, 21, 138. [Google Scholar]
- Shah, F.A.; Zeb, A.; Ali, T.; Muhammad, T.; Faheem, M.; Alam, S.I.; Saeed, K.; Koh, P.-O.; Lee, K.W.; Kim, M.O. Identification of proteins differentially expressed in the striatum by melatonin in a middle cerebral artery occlusion rat model—A proteomic and in silico approach. Front. Neurosci. 2018, 12, 888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guida, F.; Luongo, L.; Aviello, G.; Palazzo, E.; De Chiaro, M.; Gatta, L.; Boccella, S.; Marabese, I.; Zjawiony, J.K.; Capasso, R. Salvinorin A reduces mechanical allodynia and spinal neuronal hyperexcitability induced by peripheral formalin injection. Mol. Pain 2012, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hains, B.C.; Waxman, S.G. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J. Neurosci. 2006, 26, 4308–4317. [Google Scholar] [CrossRef] [Green Version]
- Zanjani, T.M.; Sabetkasaei, M.; Mosaffa, N.; Manaheji, H.; Labibi, F.; Farokhi, B. Suppression of interleukin-6 by minocycline in a rat model of neuropathic pain. Eur. J. Pharmacol. 2006, 538, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Noboru, N.; Imamura, Y.; Eliav, E. Effect of Pregabalin and Diclofenac on tactile allodynia, mechanical hyperalgesia and pro inflammatory cytokine levels (IL-6, IL-1β) induced by chronic constriction injury of the infraorbital nerve in rats. Cytokine 2018, 104, 124–129. [Google Scholar] [CrossRef] [PubMed]











| Compounds | Interleukin-6 | iNOS | COX-2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Score | H-Bond | Bonding Residues | Score | H-Bond | Bonding Residues | Score | H-Bond | Bonding Residues | |
| B3 | −9.2 | 03 | ARG 311 ILE 392 VAL395 | −6.6 | 01 | LEU 80 | −7.9 | 02 | ASN 361 HIS 212 |
| (a) | Group | GSH | GST | iNOS | LPO |
| Saline | 83.22 ± 4.2 | 73.88 ± 2.3 | 43.22 ± 1.2 | 91.13 ± 3.1 | |
| Sham | 77.26 ± 1.2 | 69.78 ± 2.4 | 46.41 ± 2.6 | 87.16 ± 3.1 | |
| CCI | 19.42 ± 2.2 ### | 17.33 ± 1.4 ### | 105.12 ± 1.7 ### | 322.26 ± 1.8 ### | |
| B3 | 55.23 ± 2.1 *** | 66.33 ± 1.0 *** | 75.20 ± 2.1 ** | 270.76 ± 2.4 ** | |
| (b) | Group | GSH | GST | iNOS | LPO |
| Saline | 66.12 ± 3.2 | 48.11 ± 1.1 | 44.15 ± 2.4 | 88.23 ± 3.3 | |
| Sham | 63.22 ± 1.2 | 49.26± 2.8 | 51.31 ± 1.0 | 78.26 ± 1.8 | |
| CCI | 8.41 ± 1.5 ### | 10.53 ± 3.4 ### | 153.82 ± 1.3 ### | 210.68 ± 1.6 ### | |
| B3 | 38.87 ± 3.5 *** | 25.73 ± 1.0 ** | 51.32 ± 3.0 *** | 121.76± 2.8 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faheem, M.; Ali, S.H.; Khan, A.W.; Alam, M.; Ilyas, U.; Zahoor, M.; Sahibzada, M.U.K.; Khalid, S.; Ullah, R.; Alqahtani, A.S.; et al. 1,3,4-Oxadiazole Derivative Attenuates Chronic Constriction Injury Induced Neuropathic Pain: A Computational, Behavioral, and Molecular Approach. Brain Sci. 2020, 10, 731. https://doi.org/10.3390/brainsci10100731
Faheem M, Ali SH, Khan AW, Alam M, Ilyas U, Zahoor M, Sahibzada MUK, Khalid S, Ullah R, Alqahtani AS, et al. 1,3,4-Oxadiazole Derivative Attenuates Chronic Constriction Injury Induced Neuropathic Pain: A Computational, Behavioral, and Molecular Approach. Brain Sciences. 2020; 10(10):731. https://doi.org/10.3390/brainsci10100731
Chicago/Turabian StyleFaheem, Muhammad, Syed Hussain Ali, Abdul Waheed Khan, Mahboob Alam, Umair Ilyas, Muhammad Zahoor, Muhammad Umar Khayam Sahibzada, Sidra Khalid, Riaz Ullah, Ali S. Alqahtani, and et al. 2020. "1,3,4-Oxadiazole Derivative Attenuates Chronic Constriction Injury Induced Neuropathic Pain: A Computational, Behavioral, and Molecular Approach" Brain Sciences 10, no. 10: 731. https://doi.org/10.3390/brainsci10100731