Abstract
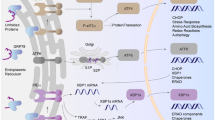
Dysfunctions of glomerular podocytes and triggering of apoptotic processes in them are the main molecular causes of diabetic nephropathy and other kidney diseases. Pathogenetic factors causing these dysfunctions of podocytes are hyperglycemia, increased levels of advanced glycation end-products, oxidative stress, increased activity of inflammatory factors, and endoplasmic reticulum stress. These factors and their combinations result in the triggering of a number of intracellular signaling pathways that cause activation of apoptosis and reduce the survival of podocytes. Among these pathways are: (1) the Wnt/β-catenin pathway, which is activated by the Wnt-proteins when they bind to the complex of Frizzled receptors and LRP co-receptors; (2) the mTOR-dependent signaling pathway, including the mTORC1 and mTORC2 complexes, which are involved in the regulation of autophagy and endoplasmic reticulum stress and are regulated by various stimuli and effectors, in particular, the AMP-activated protein kinase; (3) the Rho/ROCK signaling pathway, including GTPases of the Rho family and Rho-associated protein kinase ROCK1; (4) calcium-dependent signaling pathways triggered by an increase in the concentration of intracellular Ca2+, primarily through the activation of calcium channels of the TRPC family. The review provides a detailed analysis of the current state of knowledge about the molecular mechanisms responsible for the regulation of apoptotic processes in glomerular podocytes and also considers hormonal and other factors that regulate them both under normal conditions and under the conditions of lesions induced by hyperglycemia and diabetic nephropathy.



Similar content being viewed by others
REFERENCES
Dedov I.I., Shestakova M.V. 2000. Diabeticheskaya nefropatiya (Diabetic nephropathy), Moscow: Universum Publishing.
Lal M.A., Patrakka J. 2018. Understanding podocyte biology to develop novel kidney therapeutics. Front. Endocrinol. (Lausanne). 9, 409. https://doi.org/10.3389/fendo.2018.00409
Torban E., Braun F., Wanner N., Takano T., Goodyer P.R., Lennon R., Ronco P., Cybulsky A.V., Huber T.B. 2019. From podocyte biology to novel cures for glomerular disease. Kidney Int. 96 (4), 850–861. https://doi.org/10.1016/j.kint.2019.05.015
Dai H., Liu Q., Liu B. 2017. Research progress on mechanism of podocyte depletion in diabetic nephropathy. J. Diabetes Res. 2017, 2615286. https://doi.org/10.1155/2017/2615286
Tung C.W., Hsu Y.C., Shih Y.H., Chang P.J., Lin C.L. 2018. Glomerular mesangial cell and podocyte injuries in diabetic nephropathy. Nephrology (Carlton). 23 (Suppl. 4), 32–37. https://doi.org/10.1111/nep.13451
Lin T.A., Wu V.C., Wang C.Y. 2019. Autophagy in chronic kidney diseases. Cells. 8 (1), pii E61. https://doi.org/10.3390/cells8010061
Willert K., Nusse R. 2012. Wnt proteins. Cold Spring Harb. Perspect. Biol. 4 (9), a007864. https://doi.org/10.1101/cshperspect.a007864
Wang Y., Chang H., Rattner A., Nathans J. 2016. Frizzled receptors in development and disease. Curr. Top. Dev. Biol. 117, 113–139. https://doi.org/10.1016/bs.ctdb.2015.11.028
Jin T., George Fantus I., Sun J. 2008. Wnt and beyond Wnt: Multiple mechanisms control the transcriptional property of beta-catenin. Cell. Signal. 20 (10), 1697–1704. https://doi.org/10.1016/j.cellsig.2008.04.014
Chiang Y.T., Ip W., Jin T. 2012. The role of the Wnt signaling pathway in incretin hormone production and function. Front. Physiol. 3, 273. https://doi.org/10.3389/fphys.2012.00273
Taurin S., Sandbo N., Qin Y., Browning D., Dulin N.O. 2006. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. J. Biol. Chem. 281 (15), 9971–9976. https://doi.org/10.1074/jbc.M508778200
Zhu G., Wang Y., Huang B., Liang J., Ding Y., Xu A., Wu W. 2012. A Rac1/PAK1 cascade controls beta-catenin activation in colon cancer cells. Oncogene. 31 (8), 1001–1012 https://doi.org/10.1038/onc.2011.294
Wan J., Hou X., Zhou Z., Geng J., Tian J., Bai X., Nie J. 2017. WT1 ameliorates podocyte injury via repression of EZH2/β-catenin pathway in diabetic nephropathy. Free Radic. Biol. Med. 108, 280–299. https://doi.org/10.1016/j.freeradbiomed.2017.03.012
Guo Q., Zhong W., Duan A., Sun G., Cui W., Zhuang X., Liu L. 2019. Protective or deleterious role of Wnt/beta-catenin signaling in diabetic nephropathy: An unresolved issue. Pharmacol. Res. 144, 151–157. https://doi.org/10.1016/j.phrs.2019.03.022
Xiao L., Wang M., Yang S., Liu F., Sun L. 2013. A glimpse of the pathogenetic mechanisms of Wnt/β-catenin signaling in diabetic nephropathy. BioMed Res. Int. 2013, 7. https://doi.org/10.1155/2013/987064.987064
Bose M., Almas S., Prabhakar S. 2017. Wnt signaling and podocyte dysfunction in diabetic nephropathy. J. Investig. Med. 65 (8), 1093–1101. https://doi.org/10.1136/jim-2017-000456
Zhou L., Chen X., Lu M., Wu Q., Yuan Q., Hu C., Miao J., Zhang Y., Li H., Hou F.F., Nie J., Liu Y. 2019. Wnt/β-catenin links oxidative stress to podocyte injury and proteinuria. Kidney Int. 95 (4), 830–845. https://doi.org/10.1016/j.kint.2018.10.032
Dai C., Stolz D.B., Kiss L.P., Monga S.P., Holzman L.B., Liu Y. 2009. Wnt/β-catenin signaling promotes podocyte dysfunction and albuminuria. J. Am. Soc. Nephrol. 20 (9), 1997–2008. https://doi.org/10.1681/ASN.2009010019
Zhou T., He X., Cheng R., Zhang B., Zhang R.R., Chen Y., Takahashi Y., Murray A.R., Lee K., Gao G., Ma J.X. 2012. Implication of dysregulation of the canonical wingless-type MMTV integration site (WNT) pathway in diabetic nephropathy. Diabetologia. 55 (1), 255–266. https://doi.org/10.1007/s00125-011-2314-2
Duan S., Wu Y., Zhao C., Chen M., Yuan Y., Xing C., Zhang B. 2016. The wnt/β-catenin signaling pathway participates in rhein ameliorating kidney injury in DN mice. Mol. Cell. Biochem. 411 (1–2), 73–82. https://doi.org/10.1007/s11010-015-2569-x
Guo J., Lu C., Zhang F., Yu H., Zhou M., He M., Wang C., Zhao Z., Liu Z. 2017. VDR activation reduces proteinuria and high-glucose-induced injury of kidneys and podocytes by regulating Wnt signaling pathway. Cell. Physiol. Biochem. 43 (1), 39–51. https://doi.org/10.1159/000480315
Zhou Z., Wan J., Hou X., Geng J., Li X., Bai X. 2017. MicroRNA-27a promotes podocyte injury via PPARγ-mediated β-catenin activation in diabetic nephropathy. Cell Death Dis. 8 (3), e2658. https://doi.org/10.1038/cddis.2017.74
Wang X., Gao Y., Tian N., Zou D., Shi Y., Zhang N. 2018. Astragaloside IV improves renal function and fibrosis via inhibition of miR-21-induced podocyte dedifferentiation and mesangial cell activation in diabetic mice. Drug Des. Devel. Ther. 6 (12), 2431–2442. https://doi.org/10.2147/DDDT.S170840
Wang Y., Li H., Song S.P. 2018. β-arrestin 1/2 aggravates podocyte apoptosis of diabetic nephropathy via wnt/β-Catenin pathway. Med. Sci. Monit. 24, 1724–1732. https://doi.org/10.12659/msm.905642
Buelli S., Rosant L., Gagliardini E., Corna D., Longaretti L., Pezzotta A., Perico L., Conti S., Rizzo P., Novelli R., Morigi M., Zoja C., Remuzzi G., Bagnato A., Benigni A. 2014. β-arrestin-1 drives endothelin-1-mediated podocyte activation and sustains renal injury. J. Am. Soc. Nephrol. 25 (3), 523–533. https://doi.org/10.1681/ASN.2013040362
Liu J., Li Q.X., Wang X.J., Zhang C., Duan Y.Q., Wang Z.Y., Zhang Y., Yu X., Li N.J., Sun J.P., Yi F. 2016. β-Arrestins promote podocyte injury by inhibition of autophagy in diabetic nephropathy. Cell Death Dis. 7, e2183. https://doi.org/10.1038/cddis.2016.89
Bowin C.F., Inoue A., Schulte G. 2019. WNT-3A-induced β-catenin signaling does not require signaling through heterotrimeric G proteins. J. Biol. Chem. 294 (31), 11677–11684. https://doi.org/10.1074/jbc.AC119.009412
Bryja V., Gradl D., Schambony A., Arenas E., Schulte G. 2007. Beta-arrestin is a necessary component of Wnt/beta-catenin signaling in vitro and in vivo.Proc. Natl. Acad. Sci. USA. 104 (16), 6690–6695. https://doi.org/10.1073/pnas.0611356104
Xu H., Li Q., Liu J., Zhu J., Li L., Wang Z., Zhang Y., Sun Y., Sun J., Wang R., Yi F. 2018. β-Arrestin-1 deficiency ameliorates renal interstitial fibrosis by blocking Wnt1/β-catenin signaling in mice. J. Mol. Med. (Berl.). 96 (1), 97–109. https://doi.org/10.1007/s00109-017-1606-5
Cheng R., Ding L., He X., Takahashi Y., Ma J.X. 2016. Interaction of PPARα with the canonic wnt pathway in the regulation of renal fibrosis. Diabetes. 65 (12), 3730–3743. https://doi.org/10.2337/db16-0426
Lecarpentier Y., Claes V., Vallée A., Hébert J.L. 2017. Interactions between PPAR gamma and the canonical Wnt/beta-catenin pathway in type 2 diabetes and colon cancer. PPAR Res. 2017, 589090https://doi.org/10.1155/2017/5879090
Lecarpentier Y., Vallée A. 2016. Opposite interplay between PPAR gamma and canonical Wnt/Beta-Catenin pathway in amyotrophic lateral sclerosis. Front. Neurol. 28 (7), 100. https://doi.org/10.3389/fneur.2016.00100
Gao L., Hu Y., Li J. 2017. Pigment epithelium‑derived factor protects human glomerular mesangial cells from diabetes via NOXO1‑iNOS suppression. Mol. Med. Rep. 16 (5), 7855–7863. https://doi.org/10.3892/mmr.2017.7563
He X., Cheng R., Park K., Benyajati S., Moiseyev G., Sun C., Olson L.E., Yang Y., Eby B.K., Lau K., Ma J.X. 2017. Pigment epithelium-derived factor, a noninhibitory serine protease inhibitor, is renoprotective by inhibiting the Wnt pathway. Kidney Int. 91 (3), 642–657. https://doi.org/10.1016/j.kint.2016.09.036
Zhao C., Gao J., Li S., Liu Q., Hou X., Liu S., Xing X., Sun M., Wang S., Luo Y. 2018. Cyclin G2 suppresses glomerulosclerosis by regulating canonical wnt signalling. Biomed. Res. Int. 2018, 69382. https://doi.org/10.1155/2018/6938482
Ma S., Yao S., Tian H., Jiao P., Yang N., Zhu P., Qin S. 2017. Pigment epithelium-derived factor alleviates endothelial injury by inhibiting Wnt/β-catenin pathway. Lipids Health Dis. 16 (1), 31. https://doi.org/10.1186/s12944-017-0407-8
Chen H., Zheng Z., Li R., Lu J., Bao Y., Ying X., Zeng R., Jia W. 2010. Urinary pigment epithelium-derived factor as a marker of diabetic nephropathy. Am. J. Nephrol. 32 (1), 47–56. https://doi.org/10.1159/000314326
Bernaudo S., Salem M., Qi X., Zhou W., Zhang C., Yang W., Rosman D., Deng Z., Ye G., Yang B.B., Vanderhyden B., Wu Z., Peng C. 2016. Cyclin G2 inhibits epithelial-to-mesenchymal transition by disrupting Wnt/β-catenin signaling. Oncogene. 35 (36), 4816–4827. https://doi.org/10.1038/onc.2016.15
Wullschleger S., Loewith R., Hall M.N. 2006. TOR signaling in growth and metabolism. Cell. 124, 471–484. https://doi.org/10.1016/j.cell.2006.01.016
Torres V.E., Boletta A., Chapman A., Gattone V., Pei Y., Qian Q., Wallace D.P., Weimbs T., Wüthrich R.P. 2010. Prospects for mTOR inhibitor use in patients with polycystic kidney disease and hamartomatous diseases. Clin. J. Am. Soc. Nephrol. 5, 1312–1329. https://doi.org/10.2215/CJN.01360210
Sarbassov D.D., Ali S.M., Kim D.H., Guertin D.A., Latek R.R., Erdjument-Bromage H., Tempst P., Sabatini D.M. 2004. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14, 1296–1302. https://doi.org/10.1016/j.cub.2004.06.054
Li Q., Zeng Y., Jiang Q., Wu C., Zhou J. 2019. Role of mTOR signaling in the regulation of high glucose-induced podocyte injury. Exp. Ther. Med. 17 (4), 2495–2502. https://doi.org/10.3892/etm.2019.7236
Sakaguchi M., Isono M., Isshiki K., Sugimoto T., Koya D., Kashiwagi A. 2006. Inhibition of mTOR signaling with rapamycin attenuates renal hypertrophy in the early diabetic mice. Biochem. Biophys. Res. Commun. 340 (1), 296–301. https://doi.org/10.1016/j.bbrc.2005.12.012
Lei J., Zhao L., Zhang Y., Wu Y., Liu Y. 2018. High glucose-induced podocyte injury involves activation of mammalian target of rapamycin (mTOR)-induced endoplasmic reticulum (ER) stress. Cell. Physiol. Biochem. 45, 2431–2443. https://doi.org/10.1159/000488231
Yuan Y., Xu X., Zhao C., Zhao M., Wang H., Zhang B., Wang N., Mao H., Zhang A., Xing C. 2015. The roles of oxidative stress, endoplasmic reticulum stress, and autophagy in aldosterone mineralocorticoid receptor-induced podocyte injury. Lab. Invest. 95 (12), 1374–1386. https://doi.org/10.1038/labinvest.2015.118
Rong G., Tang X., Guo T., Duan N., Wang Y., Yang L., Zhang J., Liang X. 2015. Advanced oxidation protein products induce apoptosis in podocytes through induction of endoplasmic reticulum stress. J. Physiol. Biochem. 71 (3), 455–470. https://doi.org/10.1007/s13105-015-0424-x
Cao Y., Hao Y., Li H., Liu Q., Gao F., Liu W., Duan H. 2014. Role of endoplasmic reticulum stress in apoptosis of differentiated mouse podocytes induced by high glucose. Int. J. Mol. Med. 33 (4), 809–816. https://doi.org/10.3892/ijmm.2014.1642
Zhou H., Liu R. 2014. ER stress and hepatic lipid metabolism. Front. Genet. 5, 112. https://doi.org/10.3389/fgene.2014.00112
Zhuang A., Forbes J. M. 2014. Stress in the kidney is the road to pERdition: Is endoplasmic reticulum stress a pathogenic mediator of diabetic nephropathy? J. Endocrinol. 222 (3), R97–R111. https://doi.org/10.1530/JOE-13-0517
Appenzeller-Herzog C., Hall M.N. 2012. Bidirectional crosstalk between endoplasmic reticulum stress and mTOR signaling. Trends Cell. Biol. 22 (5), 274–282. https://doi.org/10.1016/j.tcb.2012.02.006
Hosokawa N., Hara T., Kaizuka T., Kishi C., Takamura A., Miura Y., Iemura S., Natsume T., Takehana K., Yamada N., Guan J.L., Oshiro N., Mizushima N. 2009. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol. Biol. Cell. 20 (7), 1981–1991. https://doi.org/10.1091/mbc.E08-12-1248
Hartleben B., Gödel M., Meyer-Schwesinger C., Liu S., Ulrich T., Köbler S., Wiech T., Grahammer F., Arnold S.J., Lindenmeyer M.T., Cohen C.D., Pavenstädt H., Kerjaschki D., Mizushima N., Shaw A.S., Walz G., Huber T.B. 2010. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J. Clin. Invest. 120 (4), 1084–1096. https://doi.org/10.1172/JCI39492
Inoki K., Mori H., Wang J., Suzuki T., Hong S., Yoshida S., Blattner S.M., Ikenoue T., Rüegg M.A., Hall M.N., Kwiatkowski D.J., Rastaldi M.P., Huber T.B., Kretzler M., Holzman L.B., Wiggins R.C., Guan K.L. 2011. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J. Clin. Invest. 121 (6), 2181–2196. https://doi.org/10.1172/JCI44771
Kim J., Kundu M., Viollet B., Guan K.L. 2011. AMPK and mTOR regulate autophagy through direct phosphorylation of UIk1. Nat. Cell. Bio1. 13 (2), 132–141. https://doi.org/10.1038/ncb2152
Wu L., Feng Z., Cui S., Hou K., Tang L., Zhou J., Cai G., Xie Y., Hong Q., Fu B., Chen X. 2013. Rapamycin upregulates autophagy by inhibiting the mTOR-ULK1 pathway, resulting in reduced podocyte injury. PLoS One. 8 (5), e63799. https://doi.org/10.1371/journal.pone.0066745
Xiao T., Guan X., Nie L., Wang S., Sun L., He T., Huang Y., Zhang J., Yang K., Wang J., Zhao J. 2014. Rapamycin promotes podocyte autophagy and ameliorates renal injury in diabetic mice. Mol. Cell. Biochem. 394 (1–2), 145–154. https://doi.org/10.1007/s11010-014-2090-7
Ding Y., Choi M.E. 2015. Autophagy in diabetic nephropathy. J. Endocrinol. 224 (1), R15–R30. https://doi.org/10.1530/JOE-14-0437
Wang J., Xu Z., Chen B., Zheng S., Xia P., Cai Y. 2017. The role of sirolimus in proteinuria in diabetic nephropathy rats. Iran J. Basic Med. Sci. 20 (12), 1339–1344. https://doi.org/10.22038/IJBMS.2017.9618
Liu L., Yang L., Chang B., Zhang J., Guo Y., Yang X. 2018. The protective effects of rapamycin on cell autophagy in the renal tissues of rats with diabetic nephropathy via mTOR-S6K1-LC3II signaling pathway. Ren. Fail. 40 (1), 492–497. https://doi.org/10.1080/0886022X.2018.1489287
Yang D., Livingston M.J., Liu Z., Dong G., Zhang M., Chen J.K., Dong Z. 2018. Autophagy in diabetic kidney disease: Regulation, pathological role and therapeutic potential. Cell Mol. Life Sci. 75 (4), 669–688. https://doi.org/10.1007/s00018-017-2639-1
Zhang H.T., Wang W.W., Ren L.H., Zhao X.X., Wang Z.H., Zhuang D.L., Bai Y.N. 2016. The mTORC2/Akt/NFκB pathway-mediated activation of TRPC6 participates in adriamycin-induced podocyte apoptosis. Cell. Physiol. Biochem. 40, 1079–1093. https://doi.org/10.1159/000453163
Ng T., Parsons M., Hughes W.E., Monypenny J., Zicha D., Gautreau A., Arpin M., Gschmeissner S., Verveer P.J., Bastiaens P.I., Parker P.J. 2001. Ezrin is a downstream effector of trafficking PKC-integrin complexes involved in the control of cell motility. EMBO J.20, 2723–2741. https://doi.org/10.1093/emboj/20.11.2723
Bretscher A., Edwards K., Fehon R.G. 2002. ERM proteins and merlin: Integrators at the cell cortex. Nat. Rev. Mol. Cell. Biol. 3, 586–599. https://doi.org/10.1038/nrm882
Wasik A.A., Koskelainen S., Hyvönen M.E., Musante L., Lehtonen E., Koskenniemi K., Tienari J., Vaheri A., Kerjaschki D., Szalay C., Révész C., Varmanen P., Nyman T.A., Hamar P., Holthöfer H., Lehtonen S. 2014. Ezrin is down-regulated in diabetic kidney glomeruli and regulates actin reorganization and glucose uptake via GLUT1 in cultured podocytes. Am. J. Pathol. 184, 1727–1739. https://doi.org/10.1016/j.ajpath.2014.03.002
Kawasaki Y., Imaizumi T., Matsuura H., Ohara S., Takano K., Suyama K., Hashimoto K., Nozawa R., Suzuki H., Hosoya M. 2008. Renal expression of alpha-smooth muscle actin and c-Met in children with Henoch-Schönlein purpura nephritis. Pediatr. Nephrol. 23, 913–919. https://doi.org/10.1007/s00467-008-0749-6
Ren X., Guan G., Liu G., Liu G. 2009. Irbesartan ameliorates diabetic nephropathy by reducing the expression of connective tissue growth factor and alpha-smooth-muscle actin in the tubulointerstitium of diabetic rats. Pharmacology. 83, 80–87. https://doi.org/10.1159/000180123
Carling D., Sanders M.J., Woods A. 2008. The regulation of AMP-activated protein kinase by upstream kinases. Int. J. Obes. (Lond).32 (Suppl. 4), S55–S59. https://doi.org/10.1038/ijo.2008.124
Hardie D.G. 2014. AMPK-sensing energy while talking to other signaling pathways. Cell. Metab.20 (6), 939–952. https://doi.org/10.1016/j.cmet.2014.09.013
Steinberg G.R., Carling D. 2019. AMP-activated protein kinase: The current landscape for drug development. Nat. Rev. Drug Discov.18 (7), 527–551. https://doi.org/10.1038/s41573-019-0019-2
Yan Y., Zhou X.E., Xu H.E., Melcher K. 2018. Structure and physiological regulation of AMPK. Int. J. Mol. Sci. 19 (11), E3534. https://doi.org/10.3390/ijms19113534
Tanaka Y., Kume S., Kitada M., Kanasaki K., Uzu T., Maegawa H., Koya D. 2012. Autophagy as a therapeutic target in diabetic nephropathy. Exp. Diabetes Res. 2012, 628978. https://doi.org/10.1155/2012/628978
Sharma K., RamachandraRao S., Qiu G., Usui H.K., Zhu Y., Dunn S.R., Ouedraogo R., Hough K., McCue P., Chan L., Falkner B., Goldstein B.J. 2008. Adiponectin regulates albuminuria and podocyte function in mice. J. Clin. Invest. 118 (5), 1645–1656. https://doi.org/10.1172/JCI32691
Joshi T., Singh A.K., Haratipour P., Sah A.N., Pandey A.K., Naseri R., Juyal V., Farzaei M.H. 2019. Targeting AMPK signaling pathway by natural products for treatment of diabetes mellitus and its complications. J. Cell. Physiol. 234 (10), 17212–17231. https://doi.org/10.1002/jcp.28528
Eisenreich A., Leppert U. 2017. Update on the protective renal effects of metformin in diabetic nephropathy. Curr. Med. Chem. 24 (31), 3397–3412. https://doi.org/10.2174/0929867324666170404143102
Corremans R., Vervaet B.A., D’Haese P.C., Neven E., Verhulst A. 2018. Metformin: A candidate drug for renal diseases. Int. J. Mol. Sci. 20 (1), pii: E42. https://doi.org/10.3390/ijms20010042
Blattner S.M., Hodgin J.B., Nishio M., Wylie S.A., Saha J., Soofi A.A., Vining C., Randolph A., Herbach N., Wanke R., Atkins K.B., Gyung Kang H., Henger A., Brakebusch C., Holzman L.B., Kretzler M. 2013. Divergent functions of the Rho GTPases Rac1 and Cdc42 in podocyte injury. Kidney Int. 84 (5), 920–930. https://doi.org/10.1038/ki.2013.175
Schell C., Huber T.B. 2017. The evolving complexity of the podocyte cytoskeleton. J. Am. Soc. Nephrol. 28 (11), 3166–3174. https://doi.org/10.1681/ASN.2017020143
Yu S.M., Nissaisorakarn P., Husain I., Jim B. 2018. Proteinuric kidney diseases: A podocyte’s slit diaphragm and cytoskeleton approach. Front. Med. (Lausanne). 5, 221. https://doi.org/10.3389/fmed.2018.00221
Ishizaka M., Gohda T., Takagi M., Omote K., Sonoda Y., Oliva Trejo J.A., Asao R., Hidaka T., Asanuma K., Horikoshi S., Tomino Y. 2015. Podocyte-specific deletion of Rac1 leads to aggravation of renal injury in STZ-induced diabetic mice. Biochem. Biophys. Res. Commun. 467 (3), 549–555. https://doi.org/10.1016/j.bbrc.2015.09.158
Asao R., Seki T., Takagi M., Yamada H., Kodama F., Hosoe-Nagai Y., Tanaka E., Trejo J.A.O., Yamamoto-Nonaka K., Sasaki Y., Hidaka T., Ueno T., Yanagita M., Suzuki Y., Tomino Y., Asanuma K. 2018. Rac1 in podocytes promotes glomerular repair and limits the formation of sclerosis. Sci. Rep. 8 (1), 5061. https://doi.org/10.1038/s41598-018-23278-6
Huang Z., Zhang L., Chen Y., Zhang H., Yu C., Zhou F., Zhang Z., Jiang L., Li R., Ma J., Li Z., Lai Y., Lin T., Zhao X., Zhang Q., Zhang B., Ye Z., Liu S., Wang W., Liang X., Liao R., Shi W. 2016. RhoA deficiency disrupts podocyte cytoskeleton and induces podocyte apoptosis by inhibiting YAP/dendrin signal. BMC Nephrol. 17 (1), 66. https://doi.org/10.1186/s12882-016-0287-6
Kistler A.D., Altintas M.M., Reiser J. 2012. Podocyte GTPases regulate kidney filter dynamics. Kidney Int. 81 (11), 1053–1055. https://doi.org/10.1038/ki.2012.12
Komers R. 2013. Rho kinase inhibition in diabetic kidney disease. Br. J. Clin. Pharmacol. 76 (4), 551–559. https://doi.org/10.1111/bcp.12196
Matoba K., Kawanami D., Nagai Y., Takeda Y., Akamine T., Ishizawa S., Kanazawa Y., Yokota T., Utsunomiya K. 2017. Rho-kinase blockade attenuates podocyte apoptosis by nnhibiting the Notch signaling pathway in diabetic nephropathy. Int. J. Mol. Sci. 18 (8), pii: E1795. https://doi.org/10.3390/ijms18081795
Peng F., Wu D., Gao B., Ingram A.J., Zhang B. 2008. RhoA/Rho-kinase contribute to the pathogenesis of diabetic renal disease. Diabetes. 57 (6), 1683–1692. https://doi.org/10.2337/db07-1149
Zhu L., Jiang R., Aoudjit L., Jones N., Takano T. 2011. Activation of RhoA in podocytes induces focal segmental glomerulosclerosis. J. Am. Soc. Nephrol. 22 (9), 1621–1630. https://doi.org/10.1681/ASN.2010111146
Lv Z., Hu M., Ren X., Fan M., Zhen J., Chen L., Lin J., Ding N., Wang Q., Wang R. 2016. Fyn mediates high glucose-induced actin cytoskeleton reorganization of podocytes via promoting ROCK activation in vitro.J. Diabetes Res. 2016, 5671803. https://doi.org/10.1155/2016/5671803
Lin J.S., Susztak K. 2016. Podocytes: The weakest link in diabetic kidney disease? Curr. Diab. Rep. 16 (5), 45. https://doi.org/10.1007/s11892-016-0735-5
Rao J., Ye Z., Tang H., Wang C., Peng H., Lai W., Li Y., Huang W., Lou T. 2017. The RhoA/ROCK pathway ameliorates adhesion and inflammatory infiltration induced by AGEs in glomerular endothelial cells. Sci. Rep. 7, 39727. https://doi.org/10.1038/srep39727
Wang W., Wang Y., Long J., Wang J., Haudek S.B., Overbeek P., Chang B.H., Schumacker P.T., Danesh F.R. 2012. Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab. 15 (2), 186–200. https://doi.org/10.1016/j.cmet.2012.01.009
Lin J.S., Shi Y., Peng H., Shen X., Thomas S., Wang Y., Truong L.D., Dryer S.E., Hu Z., Xu J. 2015. Loss of PTEN promotes podocyte cytoskeletal rearrangement, aggravating diabetic nephropathy. J. Pathol. 236 (1), 30–40. https://doi.org/10.1002/path.4508
Zhou J., Jia L., Hu Z., Wang Y. 2017. Pharmacological inhibition of PTEN aggravates acute kidney injury. Sci Rep. 7 (1), 9503. https://doi.org/10.1038/s41598-017-10336-8
Wang H., Feng Z., Xie J., Wen F., Jv M., Liang T., Li J., Wang Y., Zuo Y., Li S., Li R., Li Z., Zhang B., Liang X., Liu S., Shi W., Wang W. 2018. Podocyte-specific knockin of PTEN protects kidney from hyperglycemia. Am. J. Physiol. Renal Physiol. 314 (6), F1096–F1107. https://doi.org/10.1152/ajprenal.00575.2017
Buvall L., Rashmi P., Lopez-Rivera E., Andreeva S., Weins A., Wallentin H., Greka A., Mundel P. 2013. Proteasomal degradation of Nck1 but not Nck2 regulates RhoA activation and actin dynamics. Nat. Commun. 4, 2863. https://doi.org/10.1038/ncomms3863
Elvin J., Buvall L., Lindskog Jonsson A., Granqvist A., Lassén E., Bergwall L., Nyström J., Haraldsson B. 2016. Melanocortin 1 receptor agonist protects podocytes through catalase and RhoA activation. Am. J. Physiol. Renal Physiol. 310 (9), F846–F856. https://doi.org/10.1152/ajprenal.00231.2015
Sun H., Schlondorff J., Higgs H.N., Pollak M.R. 2013. Inverted formin 2 regulates actin dynamics by antagonizing Rho/diaphanous-related formin signaling. J. Am. Soc. Nephrol. 24 (6), 917–929. https://doi.org/10.1681/ASN.2012080834
Subramanian B., Sun H., Yan P., Charoonratana V.T., Higgs H.N., Wang F., Lai K.V., Valenzuela D.M., Brown E.J., Schlöndorff J.S., Pollak M.R. 2016. Mice with mutant Inf2 show impaired podocyte and slit diaphragm integrity in response to protamine-induced kidney injury. Kidney Int. 90 (2), 363–372. https://doi.org/10.1016/j.kint.2016.04.020
Zhang Y., Xia H., Ge X., Chen Q., Yuan D., Leng W., Chen L., Tang Q., Bi F. 2014. CD44 acts through RhoA to regulate YAP signaling. Cell. Signal. 26 (11), 2504–2513. https://doi.org/10.1016/j.cellsig.2014.07.031
Schwartzman M., Reginensi A., Wong J.S., Basgen J.M., Meliambro K., Nicholas S.B., D’Agati V., McNeill H., Campbell K.N. 2015. Podocyte-specific deletion of Yes-associated protein causes FSGS and progressive renal failure. J. Am. Soc. Nephrol. 27 (1), 216–226. https://doi.org/10.1681/ASN.2014090916
Bonse J., Wennmann D.O., Kremerskothen J., Weide T., Michgehl U., Pavenstädt H., Vollenbröker B. 2018. Nuclear YAP localization as a key regulator of podocyte function. Cell Death Dis. 9 (9), 850. https://doi.org/10.1038/s41419-018-0878-1
Meliambro K., Wong J.S., Ray J., Calizo R.C., Towne S., Cole B., El Salem F., Gordon R.E., Kaufman L., He J.C., Azeloglu E.U., Campbell K.N. 2017. The Hippo pathway regulator KIBRA promotes podocyte injury by inhibiting YAP signaling and disrupting actin cytoskeletal dynamics. J. Biol. Chem. 292 (51), 21137–21148. https://doi.org/10.1074/jbc.M117.819029
Kim E.Y., Alvarez-Baron C.P., Dryer S.E. 2009. Canonical transient receptor potential channel (TRPC)3 and TRPC6 associate with large-conductance Ca2+-activated K+ (BKCa) channels: Role in BKCa trafficking to the surface of cultured podocytes. Mol. Pharmacol. 75 (3), 466–477. https://doi.org/10.1124/mol.108.051912
Tian D., Jacobo S.M., Billing D., Rozkalne A., Gage S.D., Anagnostou T., Pavenstadt H., Hsu H.H., Schlondorff J., Ramos A., Greka A. 2010. Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci. Signal. 3 (145), ra77. https://doi.org/10.1126/scisignal.2001200
Greka A., Mundel P. 2011. Balancing calcium signals through TRPC5 and TRPC6 in podocytes. J. Am. Soc. Nephrol. 22 (11), 1969–1980. https://doi.org/10.1681/ASN.2011040370
Mottl A.K., Lu M., Fine C.A., Weck K.E. 2013. A novel TRPC6 mutation in a family with podocytopathy and clinical variability. BMC Nephrol. 14, 104. https://doi.org/10.1186/1471-2369-14-104
Schaldecker T., Kim S., Tarabanis C., Tian D., Hakroush S., Castonguay P., Ahn W., Wallentin H., Heid H., Hopkins C.R., Lindsley C.W., Riccio A., Buvall L., Weins A., Greka A. 2013. Inhibition of the TRPC5 ion channel protects the kidney filter. J. Clin. Invest. 123 (12), 5298–5309. https://doi.org/10.1172/JCI71165
Ilatovskaya D.V., Palygin O., Chubinskiy-Nadezhdin V., Negulyaev Y.A., Ma R., Birnbaumer L., Staruschenko A. 2014. Angiotensin II has acute effects on TRPC6 channels in podocytes of freshly isolated glomeruli. Kidney Int. 86 (3), 506–514. https://doi.org/10.1038/ki.2014.71
Ilatovskaya D.V., Levchenko V., Lowing A., Shuyskiy L.S., Palygin O., Staruschenko A. 2015. Podocyte injury in diabetic nephropathy: Implications of angiotensin II-dependent activation of TRPC channels. Sci. Rep. 5, 17637. https://doi.org/10.1038/srep17637
Ilatovskaya D.V., Staruschenko A. 2015. TRPC6 channel as an emerging determinant of the podocyte injury susceptibility in kidney diseases. Am. J. Physiol. Renal Physiol. 309 (5), F393–F397. https://doi.org/10.1152/ajprenal.00186.2015
Zhou Y., Castonguay P., Sidhom E.H., Clark A.R., Dvela-Levitt M., Kim S., Sieber J., Wieder N., Jung J.Y., Andreeva S., Reichardt J., Dubois F., Hoffmann S.C., Basgen J.M., Montesinos M.S., Weins A., Johnson A.C., Lander E.S., Garrett M.R., Hopkins C.R., Greka A. 2017. A small-molecule inhibitor of TRPC5 ion channels suppresses progressive kidney disease in animal models. Science. 358 (6368), 1332–1336. https://doi.org/10.1126/science.aal4178
Staruschenko A., Spires D., Palygin O. 2019. Role of TRPC6 in progression of diabetic kidney disease. Curr. Hypertens. Rep. 21 (7), 48. https://doi.org/10.1007/s11906-019-0960-9
Ilatovskaya D.V., Blass G., Palygin O., Levchenko V., Pavlov T.S., Grzybowski M.N., Winsor K., Shuyskiy L.S., Geurts A.M., Cowley A.W.Jr., Birnbaumer L., Staruschenko A. 2018. A NOX4/TRPC6 pathway in podocyte calcium regulation and renal damage in diabetic Kidney disease. J. Am. Soc. Nephrol. 29 (7), 1917–1927. https://doi.org/10.1681/ASN.2018030280
Spires D., Ilatovskaya D.V., Levchenko V., North P.E., Geurts A.M., Palygin O., Staruschenko A. 2018. Protective role of Trpc6 knockout in the progression of diabetic kidney disease. Am. J. Physiol. Renal Physiol.315 (4), F1091–F1097. https://doi.org/10.1152/ajprenal.00155.2018
Spires D., Manis A.D., Staruschenko A. 2019. Ion channels and transporters in diabetic kidney disease. Curr. Top. Membr. 83, 353–396. https://doi.org/10.1016/bs.ctm.2019.01.001
Rohacs T. 2013. Regulation of transient receptor potential channels by the phospholipase C pathway. Adv. Biol. Regul. 53 (3), 341–355. https://doi.org/10.1016/j.jbior.2013.07.004
Ilatovskaya D.V., Palygin O., Levchenko V., Endres B.T., Staruschenko A. 2017. The role of angiotensin II in glomerular volume dynamics and podocyte calcium handling. Sci. Rep. 7 (1), 299. https://doi.org/10.1038/s41598-017-00406-2
Kalwa H., Storch U., Demleitner J., Fiedler S., Mayer T., Kannler M., Fahlbusch M., Barth H., Smrcka A., Hildebrandt F., Gudermann T., Dietrich A. 2015. Phospholipase C epsilon (PLCε) induced TRPC6 activation: A common but redundant mechanism in primary podocytes. J. Cell. Physiol. 230 (6), 1389–1399. https://doi.org/10.1002/jcp.24883
Staruschenko A. 2019. TRPC6 in diabetic kidney disease: Good guy or bad guy? Kidney Int. 95 (2), 256–258. https://doi.org/10.1016/j.kint.2018.10.027
Smrcka A.V., Brown J.H., Holz G.G. 2012. Role of phospholipase Cε in physiological phosphoinositide signaling networks. Cell. Signal. 24 (6), 1333–1343. https://doi.org/10.1016/j.cellsig.2012.01.009
Nogueira A., Pires M.J., Oliveira P.A. 2017. Pathophysiological mechanisms of renal fibrosis: A review of animal models and therapeutic strategies. In Vivo. 31 (1), 1–22. https://doi.org/10.21873/invivo.11019
Sanz A.B., Ramos A.M., Soler M.J., Sanchez-Niño M.D., Fernandez-Fernandez B., Perez-Gomez M.V., Ortega M.R., Alvarez-Llamas G., Ortiz A. 2019. Advances in understanding the role of angiotensin-regulated proteins in kidney diseases. Expert. Rev. Proteomics. 16 (1), 77–92. https://doi.org/10.1080/14789450.2018.1545577
Sharma R., Sharma M., Vamos S., Savin V.J., Wiegmann T.B. 2001. Both subtype 1 and 2 receptors of angiotensin II participate in regulation of intracellular calcium in glomerular epithelial cells. J. Lab. Clin. Med. 138, 40–49. https://doi.org/10.1067/mlc.2001.115493
Fukuda A., Fujimoto S., Iwatsubo S., Kawachi H., Kitamura K. 2010. Effects of mineralocorticoid and angiotensin II receptor blockers on proteinuria and glomerular podocyte protein expression in a model of minimal change nephrotic syndrome. Nephrology. 15 (3), 321–326. https://doi.org/10.1111/j.1440-1797.2009.01256.x
Nijenhuis T., Sloan A.J., Hoenderop J.G., Flesche J., van Goor H., Kistler A.D., Bakker M., Bindels R.J., de Boer R.A., Moller C.C., Hamming I., Navis G., Wetzels J.F., Berden J.H., Reiser J., Faul C., van der Vlag J. 2011. Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway. Am. J. Pathol. 179 (4), 1719–1732. https://doi.org/10.1016/j.ajpath.2011.06.033
Yu Y., Zhang L., Xu G., Wu Z., Li Q., Gu Y., Niu J. 2018. Angiotensin II type I receptor agonistic autoantibody induces podocyte injury via activation of the TRPC6-calcium/calcineurin pathway in pre-eclampsia. Kidney Blood Press Res. 43 (5), 1666–1676. https://doi.org/10.1159/000494744
Nitschke R., Henger A., Ricken S., Gloy J., Müller V., Greger R., Pavenstädt H. 2000. Angiotensin II increases the intracellular calcium activity in podocytes of the intact glomerulus. Kidney Int. 57, 41–49. https://doi.org/10.1046/j.1523-1755.2000.00810.x
Kim E.Y., Anderson M., Wilson C., Hagmann H., Benzing T., Dryer S.E. 2013. NOX2 interacts with podocyte TRPC6 channels and contributes to their activation by diacylglycerol: Essential role of podocin in formation of this complex. Am. J. Physiol. Cell. Physiol. 305 (9), C960–C971. https://doi.org/10.1152/ajpcell.00191.2013
Yang Q., Wu F.R., Wang J.N., Gao L., Jiang L., Li H.D., Ma Q., Liu X.Q., Wei B., Zhou L., Wen J., Ma T.T., Li J., Meng X.M. 2018. Nox4 in renal diseases: An update. Free Radic. Biol. Med. 124, 466–472. https://doi.org/10.1016/j.freeradbiomed.2018.06.042
Gorin Y., Wauquier F. 2015. Upstream regulators and downstream effectors of NADPH oxidases as novel therapeutic targets for diabetic kidney disease. Mol. Cells. 38 (4), 285–296. https://doi.org/10.14348/molcells.2015.0010
Chiluiza D., Krishna S., Schumacher V.A., Schlondorff J. 2013. Gain-of-function mutations in transient receptor potential C6 (TRPC6) activate extracellular signal-regulated kinases 1/2 (ERK1/2). J. Biol. Chem. 288 (25), 18407–18420. https://doi.org/10.1074/jbc.M113.463059
Zhang H., Ding J., Fan Q., Liu S. 2009. TRPC6 up-regulation in Ang II-induced podocyte apoptosis might result from ERK activation and NF-kappaB translocation. Exp. Biol. Med. 234 (9), 1029–1036. https://doi.org/10.3181/0901-RM-11
Riccardi D., Valenti G. 2016. Localization and function of the renal calcium-sensing receptor. Nat. Rev. Nephrol. 12 (7), 414–425. https://doi.org/10.1038/nrneph.2016.59
Meng K., Xu J., Zhang C., Zhang R., Yang H., Liao C., Jiao J. 2014. Calcium sensing receptor modulates extracellular calcium entry and proliferation via TRPC3/6 channels in cultured human mesangial cells. PLoS One. 9 (6), e98777. https://doi.org/10.1371/journal.pone.0098777
Zhang L., Ji T., Wang Q., Meng K., Zhang R., Yang H., Liao C., Ma L., Jiao J. 2017. Calcium-sensing receptor stimulation in cultured glomerular podocytes induces TRPC6-dependent calcium entry and RhoA activation. Cell. Physiol. Biochem. 43 (5), 1777–1789. https://doi.org/10.1159/000484064
Gorvin C.M., Babinsky V.N., Malinauskas T., Nissen P.H., Schou A.J., Hanyaloglu A.C., Siebold C., Jones E.Y., Hannan F.M., Thakker R.V. 2018. A calcium-sensing receptor mutation causing hypocalcemia disrupts a transmembrane salt bridge to activate β-arrestin-biased signaling. Sci. Signal. 11 (518), eaan3714. https://doi.org/10.1126/scisignal.aan3714
Mos I., Jacobsen S.E., Foster S.R., Bräuner-Osborne H. 2019. Calcium-sensing receptor internalization is β-arrestin-dependent and modulated by allosteric ligands. Mol. Pharmacol. 96 (4), 463–474. https://doi.org/10.1124/mol.119.116772
Waasdorp M., Duitman J., Florquin S., Spek C.A. 2016. Protease-activated receptor-1 deficiency protects against streptozotocin-induced diabetic nephropathy in mice. Sci. Rep. 6, 33030. https://doi.org/10.1038/srep33030
Sharma R., Waller A.P., Agrawal S., Wolfgang K.J., Luu H., Shahzad K., Isermann B., Smoyer W.E., Nieman M.T., Kerlin B.A. 2017. Thrombin-induced podocyte injury is protease-activated receptor dependent. J. Am. Soc. Nephrol. 28 (9), 2618–2630. https://doi.org/10.1681/ASN.2016070789
Svenningsen P., Hinrichs G.R., Zachar R., Ydegaard R., Jensen B.L. 2017. Physiology and pathophysiology of the plasminogen system in the kidney. Pflugers Arch. 469 (11), 1415–1423. https://doi.org/10.1007/s00424-017-2014-y
Cunningham M.A., Rondeau E., Chen X., Coughlin S.R., Holdsworth S.R., Tipping P.G. 2000. Protease-activated receptor 1 mediates thrombin-dependent, cell-mediated renal inflammation in crescentic glomerulonephritis. J. Exp. Med. 191 (3), 455–462. https://doi.org/10.1084/jem.191.3.455
Palygin O., Ilatovskaya D.V., Staruschenko A. 2016. Protease-activated receptors in kidney disease progression. Am. J. Physiol. Renal Physiol. 311 (6), F1140–F1144. https://doi.org/10.1152/ajprenal.00460.2016
Ay L., Hoellerl F., Ay C., Brix J.M., Koder S., Schernthaner G.H., Pabinger I., Schernthaner G. 2012. Thrombin generation in type 2 diabetes with albuminuria and macrovascular disease. Eur. J. Clin. Invest. 42 (5), 470–477. https://doi.org/10.1111/j.1365-2362.2011.02602.x
Konieczynska M., Fil K., Bazanek M., Undas A. 2014. Prolonged duration of type 2 diabetes is associated with increased thrombin generation, prothrombotic fibrin clot phenotype and impaired fibrinolysis. Thromb. Haemost. 111 (4), 685–693. https://doi.org/10.1160/TH13-07-0566
Sakai T., Nambu T., Katoh M., Uehara S., Fukuroda T., Nishikibe M. 2009. Up-regulation of protease-activated receptor-1 in diabetic glomerulosclerosis. Biochem. Biophys. Res. Commun. 384, 173–179. https://doi.org/10.1016/j.bbrc.2009.04.105
Lin H., Liu A.P., Smith T.H., Trejo J. 2013. Cofactoring and dimerization of proteinase-activated receptors. Pharmacol. Rev. 65 (4), 1198–1213. https://doi.org/10.1124/pr.111.004747
Madhusudhan T., Kerlin B.A., Isermann B. 2016. The emerging role of coagulation proteases in kidney disease. Nat. Rev. Nephrol. 12 (2), 94–109. https://doi.org/10.1038/nrneph.2015.177
Li H., Wang Y., Zhou Z., Tian F., Yang H., Yan J. 2019. Combination of leflunomide and benazepril reduces renal injury of diabetic nephropathy rats and inhibits high-glucose induced cell apoptosis through regulation of NF-κB, TGF-β and TRPC6. Ren. Fail. 41 (1), 899–906. https://doi.org/10.1080/0886022X.2019.1665547
Kuang X., Zhou Q., Li Z., Hu Y., Kang Y., Huang W. 2019. –254C>G SNP in the TRPC6 gene promoter influences its expression via interaction with the NF-κB subunit RELA in steroid-resistant nephrotic syndrome children. Int. J. Genomics. 2019, 2197837. https://doi.org/10.1155/2019/2197837
Liu Y. 2004. Epithelial to mesenchymal transition in renal fibrogenesis: Pathologic significance molecular mechanism, and therapeutic intervention. J. Am. Soc. Nephrol. 15 (1), 1–12. https://doi.org/10.1097/01.asn.0000106015.29070.e7
Zhao L., Chi L., Zhao J., Wang X., Chen Z., Meng L., Liu G., Guan G., Wang F. 2016. Serum response factor provokes epithelial-mesenchymal transition in renal tubular epithelial cells of diabetic nephropathy. Physiol. Genomics. 48 (8), 580–588. https://doi.org/10.1152/physiolgenomics.00058.2016
Thiery J.P., Sleeman J.P. 2006. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 7 (2), 131–142. https://doi.org/10.1038/nrm1835
Loeffler I., Wolf G. 2015. Epithelial-to-mesenchymal transition in diabetic nephropathy: Fact or fiction? Cell. 4 (4), 631–652. https://doi.org/10.3390/cells4040631
Dai H.Y., Zheng M., Tang R.N., Ni J., Ma K.L., Li Q., Liu B.C. 2011. Effects of angiotensin receptor blocker on phenotypic alterations of podocytes in early diabetic nephropathy. Am. J. Med. Sci. 341 (3), 207–214. https://doi.org/10.1097/MAJ.0b013e3182010da9
Yamaguchi Y., Iwano M., Suzuki D., Nakatani K., Kimura K., Harada K., Kubo A., Akai Y., Toyoda M., Kanauchi M., Neilson E.G., Saito Y. Epithelial-mesenchymal transition as a potential explanation for podocyte depletion in diabetic nephropathy. Am. J. Kidney Dis. 54 (4), 653–664. https://doi.org/10.1053/j.ajkd.2009.05.009
Li Y., Kang Y.S., Dai C., Kiss L.P., Wen X., Liu Y. 2008. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am. J. Pathol.172 (2), 299–308. https://doi.org/10.2353/ajpath.2008.070057
Sun L.N., Chen Z.X., Liu X.C., Liu H.Y., Guan G.J., Liu G. 2014. Curcumin ameliorates epithelial-to-mesenchymal transition of podocytes in vivo and in vitro via regulating caveolin-1. Biomed. Pharmacother. 68 (8), 1079–1088. https://doi.org/10.1016/j.biopha.2014.10.005
Xing L., Liu Q., Fu S., Li S., Yang L., Liu S., Hao J., Yu L., Duan H. 2015. PTEN inhibits high glucose-induced phenotypic transition in podocytes. J. Cell. Biochem. 116 (8), 1776–1784. https://doi.org/10.1002/jcb.25136
Ying Q., Wu G. 2017. Molecular mechanisms involved in podocyte EMT and concomitant diabetic kidney diseases: An update. Ren. Fail. 39 (1), 474–483. https://doi.org/10.1080/0886022X.2017.1313164
Stacy A.J., Craig M.P., Sakaram S., Kadakia M. 2017. ΔNp63α and microRNAs: Leveraging the epithelial-mesenchymal transition. Oncotarget. 8 (2), 2114–2129. https://doi.org/10.18632/oncotarget.13797
Srivastava S.P., Hedayat F.A., Kanasaki K., Goodwin J.E. 2019. microRNA crosstalk influences epithelial-to-mesenchymal, endothelial-to-mesenchymal, and macrophage-to-mesenchymal transitions in the kidney. Front. Pharmacol.10, 904. https://doi.org/10.3389/fphar.2019.00904
Niu H., Nie L., Liu M., Chi Y., Zhang T., Li Y. 2014. Benazepril affects integrin-linked kinase and smooth muscle α-actin expression in diabetic rat glomerulus and cultured mesangial cells. BMC Nephrol. 15, 1–10. https://doi.org/10.1186/1471-2369-15-135
Li L., Chen L., Zang J., Tang X., Liu Y., Zhang J., Bai L., Yin Q., Lu Y., Cheng J., Fu P., Liu F. 2015. C3a and C5a receptor antagonists ameliorate endothelial-myofibroblast transition via the Wnt/β-catenin signaling pathway in diabetic kidney disease. Metabolism. 64 (5), 597–610. https://doi.org/10.1016/j.metabol.2015.01.014
Shi G., Wu W., Wan Y.G., Hex H.W., Tu Y., Han W.B., Liu B.H., Liu Y.L., Wan Z.Y. 2018. Low dose of triptolide ameliorates podocyte epithelial-mesenchymal transition induced by high dose of D-glucose via inhibiting Wnt3α/β-catenin signaling pathway activation. Zhongguo Zhong Yao Za Zhi. 43 (1), 139–146. https://doi.org/10.19540/j.cnki.cjcmm.20171027.013
Guo J., Xia N., Yang L., Zhou S., Zhang Q., Qiao Y., Liu Z. 2014. GSK-3β and vitamin D receptor are involved in β-catenin and Snail signaling in high glucose-induced epithelial-mesenchymal transition of mouse podocytes. Cell. Physiol. Biochem. 33 (4), 1087–1096. https://doi.org/10.1159/000358678
Wan J., Li P., Liu D.W., Chen Y., Mo H.Z., Liu B.G., Chen W.J., Lu X.Q., Guo J., Zhang Q., Qiao Y.J., Liu Z.S., Wan G.R. 2016. GSK-3β inhibitor attenuates urinary albumin excretion in type 2 diabetic db/db mice, and delays epithelial-to-mesenchymal transition in mouse kidneys and podocytes. Mol. Med. Rep. 14 (2), 1771–1784. https://doi.org/10.3892/mmr.2016.5441
Paeng J., Chang J.H., Lee S.H., Nam B.Y., Kang H.Y., Kim S., Oh H.J., Park J.T., Han S.H., Yoo T.H., Kang S.W. 2014. Enhanced glycogen synthase kinase-3β activity mediates podocyte apoptosis under diabetic conditions. Apoptosis. 19 (12), 1678–1690. https://doi.org/10.1007/s10495-014-1037-5
ACKNOWLEDGMENTS
The work was supported by the Russian Science Foundation (project no. 19-14-00114).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by E. Makeeva
Rights and permissions
About this article
Cite this article
Shpakov, A.O., Kaznacheyeva, E.V. Molecular Mechanisms of Apoptosis of Glomerular Podocytes in Diabetic Nephropathy. Biochem. Moscow Suppl. Ser. A 14, 205–222 (2020). https://doi.org/10.1134/S1990747820030058
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747820030058