Abstract
Background
Contribution of lipid profiles to stroke severity and outcome was inconclusive, whether chronic kidney disease (CKD) (estimated glomerular filtration rate < 60 mL/min/1.73 m2) affects the association has not been investigated. We aim to evaluate this relationship.
Methods
A retrospective study of consecutive acute ischemic stroke patients was performed. We assessed the risk of severe stroke with the National Institutes of Health Stroke Scale (NIHSS) ≥ 5 at admission and poor outcome with the modified Rankin Scale (mRS) ≥ 3 at discharge. Multivariate stepwise logistic regression models were adopted to study interaction and independent association of lipid components with stroke severity and outcome according to lipid level quartiles by CKD stratification.
Results
Among the 875 included patients (mean age 64.9 years, 67.8% males), 213 (24.3%) presented with CKD. Elevated low-density lipoprotein cholesterol (LDL-C) was independently associated with severe stroke in patients with CKD (P for trend = 0.033) than in those without CKD (P for trend = 0.121). The association between the level of LDL-C and stroke severity was appreciably modified by CKD (Pinteraction = 0.013). Compared with without CKD patients in the lowest LDL-C quartile, the multivariable-adjusted risk of severe stroke increased significantly by 2.9-fold (95% CI 1.48–5.74) in patients with CKD in the highest LDL-C quartile. No significant association was observed between lipid components and early outcome in patients with and without CKD.
Conclusion
LDL-C levels are positively associated with stroke severity in only patients with CKD, with an interactive impact of LDL-C and CKD on ischemic stroke in the acute phase.
Similar content being viewed by others
Introduction
Stroke and renal dysfunction have rapid growth trends in many countries with increasing prevalence rates of hypertension, diabetes mellitus, hyperlipidemia, and obesity [1]. The prevalence of chronic kidney disease (CKD) is estimated to be about 8–16% in the general population, and it is most common among cardiovascular disease and stroke populations [2]. Furthermore, the impact of CKD on stroke seems to be greater in Asian patients than non-Asian patients [2]. Patients with CKD often have dyslipidemia [3, 4], which is an important pathogenic factor of atherosclerosis and a modifiable risk factor for cardio- and cerebrovascular disease. Renal impairment affects blood lipid metabolism; on the other hand, dyslipidemia increases the risk of CKD [5, 6]. There was a significant effect of the interaction between lipid dyslipidemia and renal impairment.
Previous studies indicated that some lipid components were associated with increased severity of neurological injury and adverse outcomes of acute ischemic stroke (AIS) in the general population [7,8,9,10,11]. However, these studies did not stratify analysis differences between patients with and without CKD. Whether the association of lipid components with initial stroke severity and functional outcome could be modified by CKD because of a potential interaction between lipids and low estimated glomerular filtration rate (eGFR) need to be explored further. To optimize stroke management, especially in high-risk AIS populations with CKD, there is a pressing need to further assess whether serum lipid therapeutic targets in CKD patients differ from those in the general population.
The aim of this study was to investigate the association of different lipid levels with severity and functional outcome of AIS patients with and without CKD and evaluate the interaction of lipid profiles and CKD on stroke, including whether the impacts are independent of stroke etiology and other known prognosticators.
Methods
Patients
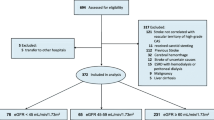
This study was a retrospective observational study of AIS patients who were consecutively admitted to the neurology department of Zhengzhou People’s Hospital (a grade A tertiary hospital) from January 2016 to May 2019. The inclusion criteria were as follows: (1) age ≥ 18 years; (2) acute ischemic stroke was confirmed by computed tomography or magnetic resonance imaging within 48 h after onset. The exclusion criteria were as follows: (1) intracerebral hemorrhage; (2) transient ischemic attack; (3) other etiologies and undetermined stroke; (4) subarachnoid hemorrhage; (5) brain tumors; (6) those treated with intravenous thrombolytic therapy before onset; (7) a modified Rankin Scale (mRS) score > 1 before onset; (8) patients with incomplete clinical data. Patients were given routine treatment for stroke with antiplatelet and anticoagulation according to etiology after admission. All data were evaluated by blindness in retrospective analysis.
Patients baseline data, including demographics, vascular risk factors (diabetes mellitus, ischemic heart disease, hypertension, atrial fibrillation, heart failure, prior stroke, smoking), blood pressure, imaging tests, lipids profile, other laboratory tests, stroke subtypes, initial stroke severity, and functional outcome, were collected and clinically evaluated. Based on clinical information, neuroimaging findings, and laboratory examinations, AIS subtypes were determined according to the Trial of Org 10,172 in Acute Stroke Treatment (TOAST) classification [12].
Biochemistry
Blood samples were taken after overnight fasting within the first 24 h of hospital admission. A TBA-FX8 biochemistry analyzer (Toshiba, Japan) was used during this study. Routine laboratory analyses, including total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), homocysteine (HCY), hemoglobin A1c (HbA1c), and serum creatinine, were performed in all enrolled patients.
Renal function measurement
Assessment of renal function was based on estimated glomerular filtration rate (eGFR) calculated using creatinine measured on the first day after admission with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation for Asians [13]. CKD was defined as an eGFR < 60 mL/min/1.73 m2.
Clinical assessment
Initial stroke severity was assessed using the National Institutes of Health Stroke Scale (NIHSS) score at admission. Severe stroke was defined as NIHSS ≥ 5. Functional outcome was evaluated by the modified Rankin Scale (mRS) score at discharge. A poor outcome was defined as mRS ≥ 3 (dependency/death).
Statistical analysis
Continuous variables were described as means ± standard deviations or medians with interquartile range (IQR). Categorical variables were presented as proportions. Student’s t or Mann-Whitney U tests were used for continuous variables, while χ2 or Fisher’s exact tests were used for categorical variables. Baseline lipid variables were further stratified into quartiles, with the lowest quartile of lipids as the reference group. We used multivariate stepwise logistic regression analysis to evaluate the association between different lipid levels and severity and outcomes of stroke by CKD stratification. The effects of interactions were examined in the models with interaction terms. Two-tailed P values < 0.05 were considered statistically significant. All analyses were performed using SPSS 25.0 (IBM, USA).
Results
Baseline characteristics
A total of 875 patients were included in the present study (mean age (64.9 ± 12.8) years, 67.8% males). Severe stroke occurred in 49.6% (434/875) of patients, poor outcomes were observed in 31.8% of patients (278/875), and 24.3% (213/875) of patients presented with CKD. Clinical characteristics are presented in detail according to CKD status in Table 1. Patients with CKD were older; were more likely to be women; smoked less; had higher systolic blood pressure levels, HCY levels, and baseline NIHSS scores; and higher rates of hypertension, ischemic heart disease, prior stroke, atrial fibrillation, and heart failure than those without CKD. Lipid profiles were not significantly different between the two groups.
Association between lipid levels and the severity of stroke by CKD status
Table 2 shows the results of the multivariate logistic regression analysis of severe stroke according to lipid level quartiles in patients with and without CKD. The age- and sex-adjusted ORs of stroke severity increased with increasing LDL-C quartiles in the groups with and without CKD (both P for trend< 0.05). A significant trend was observed in only those with CKD (P for trend = 0.033) and not in those without CKD (P for trend = 0.121) after the multivariate adjustment for relevant confounding factors. However, there was no significant association between other lipid variables and stroke severity regardless of CKD status (all Ptrend> 0.05).
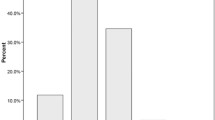
The effect of the interaction of LDL-C quartile levels and CKD on the severity of stroke is shown in Fig. 1. The association between the levels of LDL-C and stroke severity was appreciably modified by CKD status (Pinteraction = 0.013). Compared with non-CKD subjects in the lowest LDL-C quartile, the multivariable-adjusted risk of severe stroke increased significantly by 2.9-fold (95% CI: 1.48–5.74) in subjects with CKD in the highest LDL-C quartile.
The effect of the interaction of LDL-C quartile levels and CKD on the severity of stroke. Adjusted for age, sex, body mass index, systolic blood pressure, diastolic blood pressure, smoking, hypertension, diabetes mellitus, ischemic heart disease, prior stroke, atrial fibrillation, heart failure, homocysteine, hemoglobin A1c. *P = 0.002 (vs 1st Q and CKD (−))
Association between lipid levels and the outcome of stroke by CKD status
Table 3 shows the results of the logistic regression analysis of poor outcome according to lipid level quartiles in patients with and without CKD. With elevated LDL-C levels, the age- and sex-adjusted ORs of a poor outcome increased gradually in the groups without CKD (P for trend = 0.033), while HDL-C presented an inverse correlation trend in the groups with and without CKD (both P for trend< 0.05), but the significant differences disappeared after adjustment for relevant confounding factors (all P for trend> 0.05).
Discussion
In this study, we found that with elevated LDL-C levels, the severity of stroke increased. We demonstrated that only increased LDL-C levels were independently related to more severe neurological deficit in AIS patients with CKD than in those without CKD, implying that the association between LDL-C levels and stroke severity was modified by kidney function status. Data regarding the relationship between lipid levels and stroke severity have been controversial [14,15,16]. Some studies reported that low HDL-C was associated with the severity of stroke in general population [15, 16], another study argued that this association only existed in young adult stroke patients [14]. Whereas a meta-analysis showed that TG levels were linked to the severity of stroke [8]. These studies did not consider eGFR decline as an important confounder or adjusted for it in only the multivariable analysis without stratification. Whether the effects of lipid profiles on stroke in patients with CKD differ from the effect on the general population remains unclear. We founded that compared with non-CKD subjects in the lowest quartile of LDL-C, the multivariable-adjusted risk of severe stroke increased significantly by 2.9-fold (95% CI: 1.48–5.74) in subjects with CKD in the highest LDL-C quartile. There may be a significant interaction of elevated LDL-C levels and low eGFR on initial stroke severity.
Dyslipidemia exists in the early stages of CKD, aggravating renal impairment [6, 17, 18]. Lipid-modifying drugs could delay deterioration of kidney function [19, 20]. Reduced kidney function affected lipoprotein metabolism, that is, changed the composition and level of lipoproteins, and accelerated atherosclerosis [4, 21], resulting in endothelial dysfunction [22], that was associated with cardiovascular and cerebrovascular events [23,24,25]. Reduced eGFR increases stroke incidence and the prevalence of severe neurological deficits [25,26,27,28] and is a predictive factor of a poor outcome [29,30,31,32]. Recent studies revealed that the mechanisms behind this cerebro-renal interaction may be associated with a reduction in the effectiveness of dynamic cerebral autoregulation in AIS patients with a low eGFR [33]. Moreover, a low eGFR itself is also an independent risk factor for carotid atherosclerosis rather than a traditional vascular risk factor [27, 34, 35].
It is well known that the severity of stroke is the most robust determinant of outcomes in AIS patients. Patients with elevated LDL-C have severe neurological deficits in the CKD population. Whether certain lipid components were associated with functional outcome of stroke remains debatable. Putaala J et al. found that decreased HDL levels predicted adverse outcomes 3 months in young adult stroke patients [11]. In a meta-analysis with 4119 patients, TG levels were linked to stroke mortality [8], whereas a prospective study identified that there were no obvious associations between serum lipids and outcomes [15]. A multicenter clinical trial including 3348 adult patients suggested that AIS patients with high LDL-C (≥ 4.14 mmol/L) and a low eGFR calculated by CysC were independently associated with a poor 1-year outcome [36]. Our study indicated that serum lipids were not independently associated with early functional outcome regardless of eGFR status.
Our findings provide evidence for risk stratification among individuals with CKD and underscore the clinical significance of LDL-C levels as a lipid indicator of stroke severity in patients with CKD. Intensive statin treatment could effectively reduce LDL-C level and the risk of stroke in patients with CKD [37]. A large cohort prospective study confirmed that lowing LDL-C was associated with decreased stroke severity and improved functional outcomes in AIS patients [38]. It also indirectly indicated the effect of LDL-C on stroke severity. Given the detriment of elevated LDL levels in AIS patients, it may be beneficial to control LDL-C levels more strictly in patients with CKD than in those without CKD.
Some limitations should be taken into account when interpreting the results. Firstly, the retrospective observational study could not identify a definite causal relationship between LDL-C levels and stroke severity in CKD patients. Secondly, the single-center data may have exhibited the regional bias, although extensively adjusting for possible potential confounders. Thirdly, we only followed up until discharge, which may not be long enough to observe the effect of lipid profiles on outcome of AIS patients with and without CKD. There is a need for multicenter large-scale prospective studies to confirm whether a lower LDL goal is more beneficial to the long-term outcomes in CKD patients than in the general population to provide evidence for different therapeutic targets of lipids among patients with renal impairment in risk-stratified management. Notwithstanding these limitations, this is the first study of interaction and independent associations of lipid components with severity and outcome of stroke according to lipid level quartiles by CKD stratification. In addition, the eGFR was based on the CKD-EPI equation for Asians, which is considered more precise than the Modification of Diet in Renal Disease Study equation [20].
Conclusions
We present evidence on the association between elevated LDL-C levels and severe neurological deficits in AIS patients with CKD but not among those without CKD. The mechanism of pathophysiological interaction deserves intensive study.
References
Stenvinkel P (2010) Chronic kidney disease: a public health priority and harbinger of premature cardiovascular disease. J Intern Med 268(5):456–467. https://doi.org/10.1111/j.1365-2796.2010.02269.x
Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW (2013) Chronic kidney disease: global dimension and perspectives. Lancet 382(9888):260–272. https://doi.org/10.1016/S0140-6736(13)60687-X
Ferro CJ, Mark PB, Kanbay M, Sarafidis P, Heine GH, Rossignol P, Massy ZA, Mallamaci F, Valdivielso JM, Malyszko J, Verhaar MC, Ekart R, Vanholder R, London G, Ortiz A, Zoccali C (2018) Lipid management in patients with chronic kidney disease. Nat Rev Nephrol 14(12):727–749. https://doi.org/10.1038/s41581-018-0072-9
Florens N, Calzada C, Lyasko E, Juillard L, Soulage CO (2016) Modified lipids and lipoproteins in chronic kidney disease: a new class of uremic toxins. Toxins (Basel) 8(12):E376. https://doi.org/10.3390/toxins8120376
Emanuelsson F, Nordestgaard BG, Tybjaerg-Hansen A, Benn M (2019) Impact of LDL cholesterol on microvascular versus macrovascular disease: a Mendelian randomization study. J Am Coll Cardiol 74(11):1465–1476. https://doi.org/10.1016/j.jacc.2019.07.037
Zhang X, Wang B, Yang J, Wang J, Yu Y, Jiang C, Xie L, Song Y, Zhong B, Li Y, Liang M, Wang G, Li J, Zhang Y, Huo Y, Xu X, Qin X (2019) Serum lipids and risk of rapid renal function decline in treated hypertensive adults with normal renal function. Am J Hypertens 32(4):393–401. https://doi.org/10.1093/ajh/hpz001
Chen L, Xu J, Sun H, Wu H, Zhang J (2017) The total cholesterol to high-density lipoprotein cholesterol as a predictor of poor outcomes in a Chinese population with acute ischaemic stroke. J Clin Lab Anal 31(6):e22139. https://doi.org/10.1002/jcla.22139
Deng Q, Li S, Zhang H, Wang H, Gu Z, Zuo L, Wang L, Yan F (2019) Association of serum lipids with clinical outcome in acute ischaemic stroke: a systematic review and meta-analysis. J Clin Neurosci 59:236–244. https://doi.org/10.1016/j.jocn.2018.09.003
Deng QW, Li S, Wang H, Lei L, Zhang HQ, Gu ZT, Xing FL, Yan FL (2018) The short-term prognostic value of the triglyceride-to-high-density lipoprotein cholesterol ratio in acute ischemic stroke. Aging Dis 9(3):498–506. https://doi.org/10.14336/AD.2017.0629
Pikija S, Sztriha LK, Killer-Oberpfalzer M, Weymayr F, Hecker C, Ramesmayer C, Hauer L, Sellner J (2019) Contribution of serum lipid profiles to outcome after endovascular thrombectomy for anterior circulation ischemic stroke. Mol Neurobiol 56(6):4582–4588. https://doi.org/10.1007/s12035-018-1391-3
Putaala J, Strbian D, Mustanoja S, Haapaniemi E, Kaste M, Tatlisumak T (2013) Functional outcome in young adult ischemic stroke: impact of lipoproteins. Acta Neurol Scand 127(1):616–619. https://doi.org/10.1111/j.1600-0404.2012.01683.x
Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EER (1993) Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 24(1):35–41. https://doi.org/10.1161/01.str.24.1.35
Teo BW, Xu H, Wang D, Li J, Sinha AK, Shuter B, Sethi S, Lee EJ (2011) GFR estimating equations in a multiethnic Asian population. Am J Kidney Dis 58(1):56–63. https://doi.org/10.1053/j.ajkd.2011.02.393
Sanossian N, Saver JL, Kim D, Razinia T, Ovbiagele B (2006) Do high-density lipoprotein cholesterol levels influence stroke severity? J Stroke Cerebrovasc Dis 15(5):187–189. https://doi.org/10.1016/j.jstrokecerebrovasdis.2006.05.003
Tziomalos K, Giampatzis V, Bouziana SD, Spanou M, Kostaki S, Papadopoulou M, Angelopoulou SM, Tsopozidi M, Savopoulos C, Hatzitolios AI (2017) Prognostic significance of major lipids in patients with acute ischemic stroke. Metab Brain Dis 32(2):395–400. https://doi.org/10.1007/s11011-016-9924-9
Yeh PS, Yang CM, Lin SH, Wang WM, Chen PS, Chao TH, Lin HJ, Lin KC, Chang CY, Cheng TJ, Li YH (2013) Low levels of high-density lipoprotein cholesterol in patients with atherosclerotic stroke: a prospective cohort study. Atherosclerosis 228(2):472–477. https://doi.org/10.1016/j.atherosclerosis.2013.03.015
Hager MR, Narla AD, Tannock LR (2017) Dyslipidemia in patients with chronic kidney disease. Rev Endocr Metab Disord 18(1):29–40. https://doi.org/10.1007/s11154-016-9402-z
Kanda E, Ai M, Okazaki M, Yoshida M, Maeda Y (2016) Association of high-density lipoprotein subclasses with chronic kidney disease progression, atherosclerosis, and Klotho. PLoS One 11(11):e0166459. https://doi.org/10.1371/journal.pone.0166459
Taylor KS, McLellan J, Verbakel JY et al (2019) Effects of antihypertensives, lipid-modifying drugs, glycaemic control drugs and sodium bicarbonate on the progression of stages 3 and 4 chronic kidney disease in adults: a systematic review and meta-analysis. BMJ Open 9(9):e030596. https://doi.org/10.1136/bmjopen-2019-030596
Sanguankeo A, Upala S, Cheungpasitporn W, Ungprasert P, Knight EL (2015) Effects of statins on renal outcome in chronic kidney disease patients: a systematic review and meta-analysis. PLoS One 10(7):e0132970. https://doi.org/10.1371/journal.pone.0132970
Vaziri ND (2016) Disorders of lipid metabolism in nephrotic syndrome: mechanisms and consequences. Kidney Int 90(1):41–52. https://doi.org/10.1016/j.kint.2016.02.026
Kanbay M, Turgut F, Covic A, Goldsmith D (2009) Statin treatment for dyslipidemia in chronic kidney disease and renal transplantation: a review of the evidence. J Nephrology 22(5):598–609
Mahmoodi BK, Yatsuya H, Matsushita K, Sang Y, Gottesman RF, Astor BC, Woodward M, Longstreth WT Jr, Psaty BM, Shlipak MG, Folsom AR, Gansevoort RT, Coresh J (2014) Association of kidney disease measures with ischemic versus hemorrhagic strokes: pooled analyses of 4 prospective community-based cohorts. Stroke 45(7):1925–1931. https://doi.org/10.1161/STROKEAHA.114.004900
Matsushita K, Coresh J, Sang Y, Chalmers J, Fox C, Guallar E, Jafar T, Jassal SK, Landman GW, Muntner P, Roderick P, Sairenchi T, Schöttker B, Shankar A, Shlipak M, Tonelli M, Townend J, van Zuilen A, Yamagishi K, Yamashita K, Gansevoort R, Sarnak M, Warnock DG, Woodward M, Ärnlöv J, CKD Prognosis Consortium (2015) Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol 3(7):514–525. https://doi.org/10.1016/S2213-8587(15)00040-6
Masson P, Webster AC, Hong M, Turner R, Lindley RI, Craig JC (2015) Chronic kidney disease and the risk of stroke: a systematic review and meta-analysis. Nephrol Dial Transplant 30(7):1162–1169. https://doi.org/10.1093/ndt/gfv009
Lasek-Bal A, Holecki M, Kret B, Hawrot-Kawecka A, Dulawa J (2014) Evaluation of influence of chronic kidney disease and sodium disturbances on clinical course of acute and sub-acute stage first-ever ischemic stroke. Med Sci Monit 20:1389–1394. https://doi.org/10.12659/MSM.890627
Kajitani N, Uchida HA, Suminoe I, Kakio Y, Kitagawa M, Sato H, Wada J (2018) Chronic kidney disease is associated with carotid atherosclerosis and symptomatic ischaemic stroke. J Int Med Res 46(9):3873–3883. https://doi.org/10.1177/0300060518781619
Tziomalos K, Georgaraki M, Bouziana SD, Spanou M, Kostaki S, Angelopoulou SM, Papadopoulou M, Christou K, Savopoulos C, Hatzitolios AI (2017) Impaired kidney function evaluated with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation is associated with more severe acute ischemic stroke. Vasc Med 22(5):432–434. https://doi.org/10.1177/1358863X17720865
Zhang A, Xu K, Xing H, W S, Peng Q, Li F, Hang Y (2019) Effects of cardiac function and renal function on early neurological function recovery in patients with acute stroke. Chin J Neurol 52(6):463–471. https://doi.org/10.3760/cma.j.issn.1006-7876.2019.06.005
Cherng Y-G, Lin C-S, Shih C-C, Hsu Y-H, Yeh C-C, Hu C-J, Chen T-L, Liao C-C (2018) Stroke risk and outcomes in patients with chronic kidney disease or end-stage renal disease: two nationwide studies. PLoS One 13(1):e0191155. https://doi.org/10.1371/journal.pone.0191155
Khan AA, Lip GYH (2018) Role of chronic kidney disease and atrial fibrillation in outcomes of patients with ischemic stroke. Eur J Neurol 25(8):1009–1010. https://doi.org/10.1111/ene.13664
Yahalom G, Schwartz R, Schwammenthal Y, Merzeliak O, Toashi M, Orion D, Sela BA, Tanne D (2009) Chronic kidney disease and clinical outcome in patients with acute stroke. Stroke 40(4):1296–1303. https://doi.org/10.1161/STROKEAHA.108.520882
Castro P, Azevedo E, Rocha I, Sorond F, Serrador JM (2018) Chronic kidney disease and poor outcomes in ischemic stroke: is impaired cerebral autoregulation the missing link? BMC Neurol 18(1):21. https://doi.org/10.1186/s12883-018-1025-4
Chen Y-C, Su Y-C, Lee C-C, Huang Y-S, Hwang S-J (2012) Chronic kidney disease itself is a causal risk factor for stroke beyond traditional cardiovascular risk factors: a nationwide cohort study in Taiwan. PLoS One 7(4):e36332. https://doi.org/10.1371/journal.pone.0036332
Yu FP, Zhao YC, Gu B, Hu J, Yang YY (2015) Chronic kidney disease and carotid atherosclerosis in patients with acute stroke. Neurologist 20(2):23–26. https://doi.org/10.1097/NRL.0000000000000044
Zhu Z, Zhong C, Xu T, Wang A, Peng Y, Xu T, Peng H, Chen CS, Wang J, Li Q, Geng D, Sun Y, Li Y, Zhang Y, He J (2018) Prognostic significance of serum cystatin C in acute ischemic stroke patients according to lipid component levels. Atherosclerosis 274:146–151. https://doi.org/10.1016/j.atherosclerosis.2018.05.015
Yan YL, Qiu B, Wang J, Deng SB, Wu L, Jing XD, Du JL, Liu YJ, She Q (2015) High-intensity statin therapy in patients with chronic kidney disease: a systematic review and meta-analysis. BMJ Open 5(5):e006886. https://doi.org/10.1136/bmjopen-2014-006886
Fuentes B, Martinez-Sanchez P, Diez-Tejedor E (2009) Lipid-lowering drugs in ischemic stroke prevention and their influence on acute stroke outcome. Cerebrovasc Dis 27(1):126–133. https://doi.org/10.1159/000200450
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Our study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Zhengzhou People’s Hospital. Due to the retrospective nature of this study, the informed written consent was waved.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, A., Deng, W., Zhang, B. et al. Association of lipid profiles with severity and outcome of acute ischemic stroke in patients with and without chronic kidney disease. Neurol Sci 42, 2371–2378 (2021). https://doi.org/10.1007/s10072-020-04791-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-04791-x