Abstract
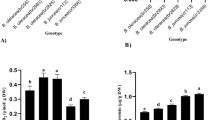
The metabolic stimulation induced by abiotic stress is an efficient strategy for the production of secondary metabolites in sterile and controlled plant cell, tissue, and organ cultures. Paclitaxel (taxol), one of the most widely used therapeutic compounds for the treatment of various cancers, is mainly produced through cell culture of the European yew (Taxus baccata L.). In this work, a T. baccata callus culture was subjected to drought stress induced in vitro by mannitol and sorbitol (1, 2, 3, and 4%) and sucrose (6 and 8%) to evaluate its impact on physiological and biochemical traits as well as paclitaxel and 10-deacetyl baccatin III (10-DBA) production. It was observed that drought stress caused a significant increase (P < 0.05) in dry weight, proline, soluble sugars, hydrogen peroxide, lipid peroxidation, total phenolics, flavonoids, and flavonols, and a decrease in relative water content, fresh weight, relative growth rate, and cell viability. Constitutive activities of superoxide dismutase, guaiacol peroxidase, ascorbate peroxidase, and glutathione reductase were enhanced by the induced stress. The content of paclitaxel and 10-DBA was higher in stressed cultures than in the control. It can be concluded that T. baccata cells have a protection mechanism against oxidative damage involving induced activities of enzymatic and non-enzymatic antioxidants. The induction of moderate drought stress could therefore be an effective strategy for increasing taxanes production in T. baccata callus cultures.






Similar content being viewed by others
References
Abdelwahd R, Hakam N, Labhilili M, Udupa SM (2008) Use of an adsorbent and antioxidants to reduce the effects of leached phenolics in in vitro plantlet regeneration of faba bean. Afr J Biotechnol 7:997–1002
Abedi T, Pakniyat H (2010) Antioxidant enzyme changes in response to drought stress in ten cultivars of oilseed rape (Brassica napus L.). Czech J Genet Plant Breed 46:27–34
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc 2:875–877
Akula R, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 6:1720–1731
Al-Khayri J, Al-Bahrany A (2002) Callus growth and proline accumulation in response to sorbitol and sucrose-induced osmotic stress in rice. Biol Plant 45:609–611
Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ 24:1337–1344
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341
Anand S (2010) Various approaches for secondary metabolite production through plant tissue culture. Pharmacia 1:1–7
Axelrod B, Cheesbrough TM, Laakso S (1981) Lipoxygenase from soybeans: EC 1.13. 11.12 Linoleate: oxygen oxidoreductase. In: Methods in enzymology, Elsevier, pp 441–451
Badawi GH, Yamauchi Y, Shimada E, Sasaki R, Kawano N, Tanaka K, Tanaka K (2004) Enhanced tolerance to salt stress and water deficit by overexpressing superoxide dismutase in tobacco (Nicotiana tabacum) chloroplasts. Plant Sci 166:919–928
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Boudet AM (2007) Evolution and current status of research in phenolic compounds. Phytochemistry 68:2722–2735
Bruňáková K, Babincova Z, Čellárová E (2004) Selection of callus cultures of Taxus baccata L. as a potential source of paclitaxel production. Eng Life Sci 4:465–469
Caverzan A, Casassola A, Brammer SP (2016) Antioxidant responses of wheat plants under stress. Genet Mol Biol 39:1–6
Cetin ES (2014) Induction of secondary metabolite production by UV-C radiation in Vitis vinifera L. Öküzgözü callus cultures. Biol Res 47:37
Chu YH, Chang CL, Hsu HF (2000) Flavonoid content of several vegetables and their antioxidant activity. J Sci Food Agric 80:561–566
Cusido RM, Onrubia M, Sabater-Jara AB, Moyano E, Bonfill M, Goossens A, Pedreño MA, Palazon J (2014) A rational approach to improving the biotechnological production of taxanes in plant cell cultures of Taxus spp. Biotechnol Adv 32:1157–1167
Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol 61:651–679
D'Cunha GB, Satyanarayan V, Nair PM (1996) Purification of phenylalanine ammonia lyase from Rhodotorula glutinis. Phytochemistry 42:17–20
de Oliveira LAR, Cardoso MN, de Oliveira ACA, de Araújo MC, Cardoso BT, da Silva AVC, da Silva LA (2018) Effects of in vitro drought stress on growth, proline accumulation and antioxidant defense in sugarcane. J Agric Sci 10:135–149
Diaz-Vivancos P, Faize M, Barba-Espin G, Faize L, Petri C, Hernández JA, Burgos L (2013) Ectopic expression of cytosolic superoxide dismutase and ascorbate peroxidase leads to salt stress tolerance in transgenic plums. Plant Biotechnol J 11:976–985
Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Dugasa MT, Cao F, Ibrahim W, Wu F (2019) Differences in physiological and biochemical characteristics in response to single and combined drought and salinity stresses between wheat genotypes differing in salt tolerance. Physiol Plant 165:134–143
Expósito O, Bonfill M, Onrubia M, Jané A, Moyano E, Cusidó RM, Palazón J, Piñol MT (2009) Effect of taxol feeding on taxol and related taxane production in Taxus baccata suspension cultures. New Biotechnol 25:252–259
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Gaafar AA, Ali SI, El-Shawadfy MA, Salama ZA, Sekara A, Ulrichs C, Abdelhamid MT (2020) Ascorbic acid induces the increase of secondary metabolites, antioxidant activity, growth, and productivity of the common bean under water stress conditions. Plants 9:627
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Gapińska M, Skłodowska M, Gabara B (2008) Effect of short-and long-term salinity on the activities of antioxidative enzymes and lipid peroxidation in tomato roots. Acta Physiol Plant 30:11–18
Ghorbanpour M, Farahani AHK, Hadian J (2018) Potential toxicity of nano-graphene oxide on callus cell of Plantago major L. under polyethylene glycol-induced dehydration. Ecotoxicol Environ Saf 148:910–922
Giannopolitis CN, Ries SK (1977) Superoxide dismutases I. Occurrence in higher plants. Plant Physiol 59:309–314
Gong M, Li YJ, Chen SZ (1998) Abscisic acid-induced thermotolerance in maize seedlings is mediated by calcium and associated with antioxidant systems. J Plant Physiol 153:488–496
Gundlach H, Müller MJ, Kutchan TM, Zenk MH (1992) Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc Natl Acad Sci 89:2389–2393
Guo Y, Yu H, Yang M, Kong D, Zhang Y (2018) Effect of drought stress on lipid peroxidation, osmotic adjustment and antioxidant enzyme activity of leaves and roots of Lycium ruthenicum Murr. Seedling. Russ J Plant Physiol 65:244–250
Hajihashemi S, Rajabpoor S, Djalovic I (2018) Antioxidant potential in Stevia rebaudiana callus in response to polyethylene glycol, paclobutrazol and gibberellin treatments. Physiol Mol Biol Plants 24:335–341
Hamanaka RB, Chandel NS (2009) Mitochondrial reactive oxygen species regulate hypoxic signaling. Curr Opin Cell Biol 21:894–899
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments: a review. Plant Signal Behav 7:1456–1466
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Jatoi SA, Latif MM, Arif M, Ahson M, Khan A, Siddiqui SU (2014) Comparative assessment of wheat landraces against polyethylene glycol simulated drought stress. Sci Technol Dev 33:1–6
Javed F, Ikram S (2008) Effect of sucrose induced osmotic stress on callus growth and biochemical aspects of two wheat genotypes. Pak J Bot 40:1487–1495
Jones AMP, Saxena PK (2013) Inhibition of phenylpropanoid biosynthesis in Artemisia annua L.: a novel approach to reduce oxidative browning in plant tissue culture. PLoS One 8:e76802
Khosroushahi AY, Naderi-Manesh H, Simonsen HT (2011) Effect of antioxidants and carbohydrates in callus cultures of Taxus brevifolia: evaluation of browning, callus growth, total phenolics and paclitaxel production. BioImpacts 1:37–45
Kim SI, Choi HK, Kim JH, Lee HS, Hong SS (2001) Effect of osmotic pressure on paclitaxel production in suspension cell cultures of Taxus chinensis. Enzym Microb Technol 28:202–209
Kim TH, Böhmer M, Hu H, Nishimura N, Schroeder JI (2010) Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ Signaling. Annu Rev Plant Biol 61:561–591
Król A, Amarowicz R, Weidner S (2014) Changes in the composition of phenolic compounds and antioxidant properties of grapevine roots and leaves (Vitis vinifera L.) under continuous of long-term drought stress. Acta Physiol Plant 36:1491–1499
Li Y, Kong D, Fu Y, Sussman MR, Wu H (2020) The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol Biochem 148:80–89
Lila MA (2004) Valuable secondary products from in vitro culture, Plant Development and Biotechnology, CRC Press, pp 285–292
Liu X, Huang B (2000) Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass. Crop Sci 40:503–510
Liu H, Wang X, Wang D, Zou Z, Liang Z (2011) Effect of drought stress on growth and accumulation of active constituents in Salvia miltiorrhiza Bunge. Ind Crop Prod 33:84–88
Maehly A, Chance B (1954) Catalases and peroxidases. Methods Biochem Anal 1:357–424
Malik S, Cusidó RM, Mirjalili MH, Moyano E, Palazón J, Bonfill M (2011) Production of the anticancer drug taxol in Taxus baccata suspension cultures: a review. Process Biochem 46:23–34
Mehdy MC (1994) Active oxygen species in plant defense against pathogens. Plant Physiol 105:467–472
Miliauskas G, Venskutonis P, Van BT (2004) Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem 85:231–237
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7: 405– 410
Mohanty N (2003) Photosynthetic characteristics and enzymatic antioxidant capacity of flag leaf and the grain yield in two cultivars of Triticum aestivum (L.) exposed to warmer growth conditions. J Plant Physiol 160:71–74
Mutava RN, Prince SJK, Syed NH, Song L, Valliyodan B, Chen W, Nguyen HT (2015) Understanding abiotic stress tolerance mechanisms in soybean: a comparative evaluation of soybean response to drought and flooding stress. Plant Physiol Biochem 86:109–120
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Oh MM, Trick HN, Rajashekar C (2009) Secondary metabolism and antioxidants are involved in environmental adaptation and stress tolerance in lettuce. J Plant Physiol 166:180–191
Onrubia M, Moyano E, Bonfill M, Cusidó RM, Goossens A, Palazón J (2013) Coronatine, a more powerful elicitor for inducing taxane biosynthesis in Taxus media cell cultures than methyl jasmonate. J Plant Physiol 170:211–219
Osuna L, Tapia N, Cusidó R, Palazón J, Bonfill M, Zamilpa A, López-Upton J, Cruz-Sosa F (2015) Taxane production induced by methyl jasmonate in free and immobilized cell cultures of Mexican yew (Taxus globosa Schltdl). Acta Physiol Plant 37:199
Razavizadeh R, Farahzadianpoor F, Adabavazeh F, Komatsu S (2019) Physiological and morphological analyses of Thymus vulgaris L. in vitro cultures under polyethylene glycol (PEG)-induced osmotic stress. In Vitro Cell Dev Biol Plant 55:342–357
Roberts SC, Shuler ML (1997) Large-scale plant cell culture. Curr Opin Biotechnol 8:154–159
Sabater-Jara A, Tudela L, López-Pérez A (2010) In vitro culture of Taxus sp.: strategies to increase cell growth and taxoid production. Phytochem Rev 9:343–356
Sahraroo A, Mirjalili MH, Corchete P, Babalar M, Moghadam MRF (2016) Establishment and characterization of a Satureja khuzistanica Jamzad (Lamiaceae) cell suspension culture: a new in vitro source of rosmarinic acid. Cytotechnology 68:1415–1424
Sairam R, Srivastava G (2002) Changes in antioxidant activity in sub-cellular fractions of tolerant and susceptible wheat genotypes in response to long term salt stress. Plant Sci 162:897–904
Sairam RK, Rao KV, Srivastava G (2002) Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci 163:1037–1046
Shirshekan M, Rezadoost H, Javanbakht M, Ghassempour AR (2017) The combination process for preparative separation and purification of paclitaxel and 10-deacetylbaccatin III using Diaion® Hp-20 followed by hydrophilic interaction based solid phase extraction. Iran J Pharm Res 16:1396–1404
Smetanska I (2008) Production of secondary metabolites using plant cell cultures. In: Food biotechnology, Springer, pp 187–228
Smirnoff N (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125:27–58
Solecka D (1997) Role of phenylpropanoid compounds in plant responses to different stress factors. Acta Physiol Plant 19:257–268
Soliva RC, Elez P, Sebastián M, Martín O (2000) Evaluation of browning effect on avocado purée preserved by combined methods. Innov Food Sci Emerg Technol 1:261–268
Sun RL, Zhou QX, Sun FH, Jin CX (2007) Antioxidative defense and proline/phytochelatin accumulation in a newly discovered Cd-hyperaccumulator, Solanum nigrum L. Environ Exp Bot 60:468–476
Thoma I, Loeffler C, Sinha AK, Gupta M, Krischke M, Steffan B, Roitsch T, Mueller MJ (2003) Cyclopentenone isoprostanes induced by reactive oxygen species trigger defense gene activation and phytoalexin accumulation in plants. Plant J 34:363–375
Titov S, Bhowmik SK, Mandal A, Alam MS, Uddin SN (2006) Control of phenolic compound secretion and effect of growth regulators for organ formation from Musa spp. cv. Kanthali floral bud explants. Am J Biochem Biotechnol 2:97–104
Tiwari R, Rana CS (2015) Plant secondary metabolites: a review. Int J Eng Res Gen Sci 3:661–670
Vaughn KC, Duke SO (1984) Function of polyphenol oxidase in higher plants. Physiol Plant 60:106–112
Velioglu Y, Mazza G, Gao L, Oomah B (1998) Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem 46:4113–4117
Vidal-Limon HR, Almagro L, Moyano E, Palazon J, Pedreño MA, Cusido RM (2018) Perfluorodecalins and hexenol as inducers of secondary metabolism in Taxus media and Vitis vinifera cell cultures. Front Recent Dev Plant Sci 9:335
Vogt T (2010) Phenylpropanoid biosynthesis. Mol Plant 3: 2–20
Wang D, Du F, Liu H, Liang Z (2010) Drought stress increases iridoid glycosides biosynthesis in the roots of Scrophularia ningpoensis seedlings. J Med Plant Res 4:2691–2699
Wu J, Ge X (2004) Oxidative burst, jasmonic acid biosynthesis, and taxol production induced by low-energy ultrasound in Taxus chinensis cell suspension cultures. Biotechnol Bioeng 85:714–721
Yu LJ, Lan WZ, Chen C, Yang Y, Sun YP (2005) Importance of glucose-6-phosphate dehydrogenase in taxol biosynthesis in Taxus chinensis cultures. Biol Plant 49:265–268
Yu LJ, Lan WZ, Qin WM, Xu HB (2002a) High stable production of taxol in elicited synchronous cultures of Taxus chinensis cells. Process Biochem 38:207–210
Yu LJ, Lan WZ, Qin WM, Jin WW, Xu HB (2002b) Oxidative stress and taxol production induced by fungal elicitor in cell suspension cultures of Taxus chinensis. Biol Plant 45:459–461
Zhang C, Fevereiro PS (2007) The effect of heat shock on paclitaxel production in Taxus yunnanensis cell suspension cultures: role of abscisic acid pretreatment. Biotechnol Bioeng 96:506–514
Zhang Y, Yang J, Lu S, Cai J, Guo Z (2008) Overexpressing SgNCED1 in tobacco increases ABA level, antioxidant enzyme activities, and stress tolerance. J Plant Growth Regul 27:151–158
Zhong JJ (2002) Plant cell culture for production of paclitaxel and other taxanes. J Biosci Bioeng 94:591–599
Acknowledgements
The authors express their appreciation to the Research Council of Razi University, Kermanshah, Iran, Shahid Beheshti University, Tehran, Iran, and the University of Barcelona, Spain, for their financial support. We also wish to thank Miss. Zahra Aminfar and Mr. Hamid Ahadi for their kind help in data collection and taxanes analysis, respectively. This work is part of Marziyeh Sarmadi PhD’s thesis.
Funding
This study received financial support from the Research Council of Razi University, Kermanshah, Iran, Shahid Beheshti University, Tehran, Iran, and the University of Barcelona, Spain.
Author information
Authors and Affiliations
Contributions
MS contributed to the conception of the study, in vitro cultures establishment, statistical analysis, and interpretation of data, and wrote the first draft of the manuscript. NK supervised the study and revised the manuscript. AGh carried out the HPLC analysis. JP hosted MS at the University of Barcelona for a short research stay and carried out biochemical analysis and revised the manuscript as well. MHM supervised the whole experiments and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editor: Yong Eui Choi
Rights and permissions
About this article
Cite this article
Sarmadi, M., Karimi, N., Palazón, J. et al. Physiological, biochemical, and metabolic responses of a Taxus baccata L. callus culture under drought stress. In Vitro Cell.Dev.Biol.-Plant 56, 703–717 (2020). https://doi.org/10.1007/s11627-020-10128-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-020-10128-2