Abstract
Valuation of water quality index (WQI) is one of the simplest, easily understandable, and efficacious techniques to evaluate the quality and suitability of water for drinking as well as other purposes. This research was aimed to investigate the drinking water quality of tube wells from different areas in Khulna City, Bangladesh, by developing the WQI. Water samples from 59 tube wells were collected from different locations during the pre-monsoon time. pH, electric conductivity (EC), dissolve oxygen (DO), total dissolved solid (TDS), chloride (Cl−), nitrate (NO3−), and total hardness of the collected water samples were analyzed for the calculation of WQI. The mean value for pH, EC, DO, TDS, Cl−1, NO3−, and total hardness was 7.30, 1650 μS/cm, 1.60 mg/l, 1188.7 mg/l, 414.6 mg/l, 0.029 mg/l, and 52.03 mg/l, respectively. The calculated WQI values for individual places were distributed spatially through mapping by using ArcGIS software. Based on the WQI values, the drinking water was categorized into excellent, good, poor, very poor, and unfit for drinking purposes. The calculated WQI values ranged from 40.11 to 454.37 with an average value of 108.94. Among all the groundwater samples, 11.86% were excellent, 54.24% were good, 23.73% were poor, 1.69% were very poor, and 8.47% were unfit for drinking purpose based on WQI. The results showed that the groundwater quality of most of the studied areas of Khulna city could be considered safe and suitable for drinking barring the elevated EC and chloride content in some areas. Since Khulna city is situated in the southwestern part of Bangladesh and gradually approaches toward the base level of the Bay of Bengal which might be the source of salt concentration in the groundwater of Khulna city, Bangladesh.
Similar content being viewed by others
Introduction
From the dawn of earth to the present, water is one of the essential factors that contribute to sustaining every form of life. Due to the growing demand for water worldwide, the assurance of adequate supply of drinking water by maintaining standard water quality has become a significant challenge (Mekonnen and Hoekstra 2016; Sawyer et al. 2003). Among the pure sources of water, groundwater (GW) is the most reliable and most extensive storage of freshwater on earth which is used by one-third of the entire world’s population for drinking purposes (Foster and Chilton 2003; Nickson et al. 2005). Despite the significance of GW, the quality of this natural resource is not maintained correctly. Various pollution sources, climate changes, GW recharge, subsurface geochemical reactions, surface water characteristics, geographical locations, atmospheric precipitation, and anthropogenic activities are the most critical factors that affect the quality and quantity of GW directly and indirectly (Collin and Melloul 2003; Kumar et al. 2014; Ramakrishnaiah et al. 2009). Furthermore, the intrusion of saline water into the coastal area is one of the leading causes responsible for the deterioration of GW quality (Karro et al. 2004; Kim et al. 2006).
Salinity in GW has become a significant problem in the south-west coastal region of Bangladesh, including Khulna City, located on the bank of Bhairab and Rupsa river. This city is gradually inclined to the base level of the Bay of Bengal (Hoque et al. 2003; Woobaidullah et al. 2006). Here, some areas are facing enormous challenges to meet the increasing demand for freshwater due to the intrusion of excess saline water which impedes the supply of fresh drinking water and has become a very alarming issue for the city dwellers. The excess extraction of GW from the aquifer for drinking and irrigation purposes decreases the freshwater supply by lowering the GW table and eventually sea water enters into the GW zone to fill up the gap. Excess salt concentration also changes other water quality parameters and alters the natural composition of water (Abdalla et al. 2010; Adhikary et al. 2012; Van Camp et al. 2014). Side by side, continuous use of polluted water results in a detrimental effect on human health and socio-economic development (Milovanovic 2007). So, to control the use of polluted water especially for drinking purposes, regular monitoring of the GW quality is a very compatible step in recent times (Hasan et al. 2019; Saeedi et al. 2010).
For evaluating the water quality, estimation of WQI is one of the most suitable techniques which was first suggested and developed by Horton (1965). Usually, WQI is calculated to assess the water quality by considering its suitability for drinking purposes mainly. So, it has become a handy tool for the management of GW quality and ensures the utilization of pure drinking water (Ramakrishnaiah et al. 2009; Tiwari et al. 2014). This procedure helps to express the water quality stably and simply because this tool is very efficient to transform large quantities of complex data into a single number. Thus, the values derived from WQI can be considered as much more convenient and easily understandable to the researchers, general audiences, concerned citizens as well as national water policymakers for management and decision-making purposes. Though there are different ways of calculating WQI each system considers similar physical and chemical parameters of water and the only differences among the procedures are: the way of integration of the data and interpretation of the obtained results (Reza and Singh 2010; Sinha et al. 2004; Stambuk-giljanovic 1999). The computed data were displayed by using a GIS mapping system, which is a very convenient and powerful tool that provides a guideline to understand, interpret and visualize the data easily through modeling or graphical presentation systems (Gupta and Srivastava 2010; Krishnaraj et al. 2015).
The present research work was aimed to investigate the quality and to justify the suitability of tube well water of different areas in Khulna City, Bangladesh for drinking purpose by calculating WQI; and to project the status of physicochemical parameters and WQI by preparing a proper map through GIS.
Materials and methods
Study area
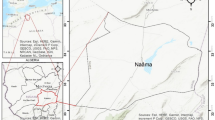
The experiment was carried out in Khulna City, the third biggest city in Bangladesh. The city is situated in the southwestern part of the country, covering an area of approximately 59.57 square kilometres (Wikipedia 2020). The latitude and longitude of the city are 22° 46′ to 22° 58′ North and 89° 28′ to 89° 37′ East, respectively. The city is located on a riverine area where the tidal river Rupsa crosses the middle part of the city, the river Bhairab is on the northern, the Pasur river is on the southern side, and the Mayur river is located in the western area of the city (Adhikary et al. 2012; Roy et al. 2005).
According to Khulna Water Supply and Sewerage Authority (KWASA), the number of total tube wells in Khulna city was approximately 55 in 1996, then increased to 111 in 2011, and now the number is more (IWM 2011). In this experiment, a total of 59 water samples was collected from 59 different locations (Fig. 1). The sampling sites were predetermined and selected in such a way that the main populated areas of Khulna City were covered irrespective of the depth of the tube wells. Moreover, to ensure the permanent drinking water reservoir site, tube wells installed in schools, colleges, and religious places were selected as groundwater sources. The longitude and latitude values of the sample sites (Table 1) also denoted to specify the locations.
Water sampling
Water samples were collected from selective tube wells with intensive care. After continuous pumping of the tube well for 5 min, water samples were collected in new plastic bottles after washing the bottle properly to avoid any unwanted contamination of water. The bottles were filled up entirely, and the caps were closed very carefully to create the airtight condition. No air bubble was seen inside the bottle which ensured the absence of any trapped air in the water, and that helped to prevent the oxidation of the reduced substances of water samples during the time of transportation and storage. After collection, samples were immediately stored in a closed icebox. Then, the samples were brought to the laboratory for chemical analysis, and different parameters were analyzed as soon as possible to avoid different chemical and biological reactions that may take place over time and change the water quality. Side by side, for some time-consuming, complex analysis, and samples were stored in a refrigerator.
Chemical analysis of water samples
pH, EC, and TDS were measured by using a portable multimeter (Hanna HI9813-5 Portable pH/EC/TDS Meter). Before taking the reading, the meter was calibrated by using a calibration solution for a particular analysis. DO was determined by using DO Meter (HI98193). A zero oxygen solution (HI7040) was used to calibrate the probe of the device. The chloride of the water sample was determined by the titrimetric method (Mohr’s Argentometric method, APHA 4500 Cl-B) where potassium was used to determine the concentration of chloride ions in solution (Mohr 1856). Total hardness was determined by estimating the Ca and Mg content of water samples through complexometric titration with EDTA, where Eriochrome Black T was used as the indicator of the visual endpoint (Harris 2010). Nitrate was determined by following the prescribed method of Cataldo et al. (1975).
The procedure of WQI estimation
WQI was calculated by following four steps. In step 1, according to the relative importance of each parameter (pH, EC, TDS, DO, chloride, nitrate, and total hardness) in the quality measurement criteria of water for drinking purpose, a particular weight (wi) was assigned for each parameter (Table 2). EC, TDS, chloride, and nitrate were considered as the key elements, and the maximum weight (5.0) was set for each of those (Vasanthavigar et al. 2010). pH and DO were categorized under the weight of 4.0 according to its value on water quality determination. As total hardness (calcium and magnesium) is considered less important than the other parameters in measuring drinking water quality, minimum weight (2.0) was assigned for it (Batabyal and Chakraborty 2015).
In step 2, the relative weight (Wi) was calculated by using the following equation (eq):
Here Wi the relative weight; wi the weight of each parameter; and n the total number of parameters.
Next, for each parameter, a quality rating scale (qi) was computed in step 3, which was obtained by dividing the concentration of each water sample by the respective standard of particular parameter mentioned in the guideline of Bureau of Indian Standards value, BIS 10500 (1991) and then the acquired result was multiplied by 100 (Batabyal and Chakraborty 2015; BIS 1991). So, the equation of qi:
Here qi quality rating of each parameter; Ci concentration (mg/l) of each chemical parameter in water sample; and Si Bureau of Indian Standards value for each chemical parameter in mg/l.
In the last step (step-4), at first SI for each chemical parameter was determined, and then the value the SI was used to calculate WQI by using the following equation:
where SIi sub-index of the ith parameter; qi quality rating of the ith parameter; and n the number of parameters.
Classification of WQI range
The obtained WQI values were categorized under five categories. The ranges of WQI values and water categories are mentioned in Table 3.
Preparation of map
Several maps were prepared to delineate the spatial distribution of physicochemical parameters and WQI of groundwater quality by using GIS ArcGIS-version 10.5 software.
Results and discussion
General information and physicochemical data
The depth of tube wells and estimated values of the necessary parameters (pH, DO, EC, TDS, chloride, nitrate, and total hardness) to determine the WQI are given in Table 4. The pH value is one of the most vital indices that denotes the acidity or alkalinity and hydrogen ion concentration of groundwater (Murugesan et al. 2006). In our experiment, the pH value of the groundwater samples ranged from 6.40 to 7.90 with a mean value of 7.30 (Table 4) which indicates that water of the experimental areas was in optimal condition for drinking purpose as the values can be categorized within the permissible range (6.5–8.5) of drinking water according to WHO (2011). Akter et al. (2016) found the mean pH value of 7.4 in the drinking water of the Khulna area. Sikder et al. (2014) also observed an average pH value of 7.89 in the pre-monsoon season in Khulna coastal belt areas.
The total concentration of soluble salt, in the sampled water, expressed by EC, is one of the most prominent parameters to measure WQI, especially in coastal or salinity prone areas. EC value in this study varied from 100 to 6830 μS/cm with an average value of 1650 μS/cm (Table 4), and around 37% water samples in Khulna City was included in the maximal allowable limit of EC (1000 μS/cm) given by BIS (1991) and NDWQS (2004) which presents the high amount of salts in the groundwater of the selected areas. The spatial distribution of sampling units could explain this wide variation in EC values. It was observed that some sampling units were situated very close to the tidal river (Fig. 1) and it was confirmed from Table 4 that the water collected from those points contained higher values of EC. The higher EC values in the groundwater studied in this research might be due to enrichment of salts in the water through saltwater intrusion since the areas are located near the coast as well as tidal rivers flow across the city. It might also be because of the interaction of rock and water and agricultural activities (Abbasnia et al. 2019). Sikder et al. (2014) experimented with assessing the groundwater quality of the western area of Khulna in the pre-monsoon season and obtained EC value of drinking water ranged from 789 to 1230 μS/cm, whereas Adhikary et al. (2012) observed an average EC value of 1777 μS/cm by researching six wards of Khulna City Corporation.
The calculated TDS values of groundwater samples fluctuated from 7.240 to 6450.0 mg/l, and the average value was 1188.7 mg/l (Table 4). Around 32% of the total samples exceeded the admissible limits (1000 mg/l) of TDS recommended by WHO (2006). Therefore, the water quality for the selected tube wells in this study was categorized as brackish (1000 < TDS < 10,000 mg/L) (Logeshkumaran et al. 2015; Abbasnia et al. 2019). TDS in the groundwater studied in this research was high due to enrichment of salts in the water. It might also be because of the contact between rock and water and agricultural activities (Abbasnia et al. 2019). An average value of 1043 mg/l TDS in Khulna (Adhikary et al. 2012) and an average value of 1556.05 mg/l (Islam et al. 2017) in the groundwater of the coastal aquifer of Khulna city were also reported. Percolation of elements from rocks and gypsum rocks might be another reason for high EC and TDS in the water. Excessive withdrawal of groundwater, as well as excessive use of chemical fertilizers in the agricultural field contributing the higher concentration of ions into the groundwater, might be the other reasons for decreasing the water quality in terms of EC and TDS (Abbasnia et al. 2019).
Analysis of DO concentration in water is a significant step for WQI estimation, as oxygen is one of the essential regulatory factors that control the metabolic process of living substances in water and act as an indicator of the condition of water (Basavaraddi et al. 2012). The estimated maximum, minimum, and average values of DO were 4.21, 0.59, and 1.60 mg/l, respectively (Table 4). The estimated values were less than the standard limit of DO 6.0 mg/l, which is suggested by DPHE (2018) and ECR (1997). Sikder et al. (2014) also found a lower average DO value of 3.07 mg/l in the pre-monsoon season in Khulna. That means the observed results from different studies indicate that the condition of DO in this region is not at a satisfactory level and much lower than the standard condition.
The quantity of chloride concentration in the present study was in the range of 35.50–414.6 mg/l. The average value was 2769 mg/l (Table 4) indicating a higher value of chloride and around 47% of the sampled water exceeded the permissible limit of chloride in drinking water which is 250 mg/l according to WHO (2006), and more than 64% of samples surpassed the acceptable limit (150 mg/l to 600 mg/l) mentioned in ECR (1997) of Bangladesh standard. The presence of high-chloride concentration in the groundwater might be due to contact between water, and soil and rock; and weathering as well as owing to anthropogenic contribution like effluent of wastewaters (Abbasnia et al. 2018). The higher amount of chloride (1776.74 mg/l) in shallow groundwater aquifer in the coastal region of Khulna was reported by Islam et al. (2017). Adhikary et al. (2012) also showed similar results of high chloride content in the groundwater of the Khulna region.
The total hardness of water represents the total dissolved calcium and magnesium content in water, is also an essential factor that helps to determine the suitability of water for drinking purpose (Howladar et al. 2018). The total hardness of the water in the Khulna city area changed from 16.00 to 178.8 mg/l, and the mean value was 52.03 mg/l (Table 4). The values lie under the allowable limit of hardness according to Bangladeshi standard, 200–500 mg/l (ECR, 1997), and WHO standard, 500 mg/l (WHO 2006). So, the total hardness of the sampled water was satisfactory for drinking purposes. However, Adhikary et al. (2012) observed a higher value of total hardness (641 mg/l) from the groundwater sample of six wards of Khulna City Corporation, Bangladesh.
The amount of nitrate in the groundwater was minimum and under the allowable value of 10 mg/l and 45 mg/l, given by Bangladesh standard, ECR (1997), and WHO (2011), respectively. The results revealed that the nitrate concentration was in the range of 0.034–0.029 mg/l and the average value of nitrate was 0.026 mg/l (Table 4). Lower quantities of nitrate in the water sample of the Khulna region were also reported (Islam et al. 2017; Sikder et al. 2014).
The spatial analysis map of the depth of tube wells and physicochemical parameters of water
Depth wise spatial distribution of tube wells and physicochemical parameters of the water samples in the experimental areas of Khulna City are presented in Figs. 2, 3, 4, 5, 6, 7, 8, 9. The region covered by numerous water types is calculated from the WQI maps and given in Fig. 10. From the maps, it was clearly shown that the values of physicochemical parameters varied from place to place irrespective of the depth of the tube wells.
Water quality index (WQI)
The value of WQI of the sampled water with respective categories of each water sample is presented in Table 4. In the present experiment, the computed values of WQI ranged from 40.11 to 454.37, and the average value was 108.94. Here, lower values of WQI (< 50) were excellent and higher values (> 300) were unfit for drinking purposes (Table 3). Among all the groundwater samples, 11.9% of groundwater samples were “excellent”, 54.2% were “good”, 23.7% were “poor”, 1.7% were “very poor”, and 8.5% were “unfit for drinking purpose” based on WQI. So, more than half of the location falls in excellent to good categories. A suitable diagram was prepared (Fig. 10) by spatial analysis system for clear visualization of the areas which are categorized under different water quality parameters by using the WQI values of 59 sampling points in Khulna city of Bangladesh. In the GIS map, the five categories were delineated with different color, and each color represents the information about the spatial distribution of water of varying quality. From the map, it is visualized that, the tube wells of the south-east area of Khulna city supply better quality drinking water than those of the north-west side. It is also mentionable that the WQI values were not compatible with the values of depth of tube wells.
One vital fact is that there is no mentionable published research work to identify the suitability of groundwater for drinking purposes through investigating the WQI in the Khulna City area. So, it was not possible to compare our experimental data directly with any other studies. However, our findings were quite similar to the results of Akter et al. (2016) who conducted research to estimate different water quality parameters and WQI of different areas of Bangladesh including Khulna as a coastal area and observed that majority of groundwater sources were under the good category of drinking water. Though a considerable percentage of groundwater sources were suitable for drinking purpose, a significant number of tube wells supplies poor quality drinking water. It is conceivable that the higher values of EC, TDS, and chloride in the groundwater were the prominent factors of the higher values of WQI which indicates poor drinking water quality indicating high salinity of the water. Due to being a coastal area, the intrusion of saline water in some regions of Khulna can be considered as one of the critical reasons for the deterioration of water quality for drinking purpose in those areas (Adhikary et al. 2012; Akter et al. 2016).
Conclusion
The suitability of the groundwater, as a significant source of drinking purposes, was investigated in the Khulna city, the southwestern part of Bangladesh. WQI is the valuable and sole rating to describe the overall water quality status in a single term. However, WQI depicts the combined effect of different water quality parameters and transfers water quality information to the public and government decision-makers. WQI is a single value to represent the water quality of a source along with decreasing the larger number of parameters into a simple countenance resulting in easy understanding of water quality observing data. This present research work demonstrated the analytical data to assess the water quality and the utility of GIS, which combinedly represent the WQI of 59 selected tube wells of Khulna city area in Bangladesh through mapping. The WQI for groundwater samples ranged from 40.11 to 454.37, with an average value was 108.94. More than 60% of the samples were in the good and excellent category in water quality assessment, suitable for drinking water, and the other 40% of the samples were needed to be treated before using drinking purposes. The spatial distribution map of DO, EC, TDS, pH, chloride, nitrate, and total hardness showed that these parameters were not distributed uniformly throughout the study area. The high value of WQI at these samples had been found mainly from the higher values of salinity problem (high EC, TDS, and chloride) in the groundwater which might be due to seawater intrusion since Khulna city is situated in the coastal region of Bangladesh.
References
Abbasnia A, Yousefi N, Mahvi AH, Nabizadeh R, Radfard M, Yousefi M, Alimohammadi M (2019) Evaluation of groundwater quality using water quality index and its suitability for assessing water for drinking and irrigation purposes: case study of Sistan and Baluchistan province (Iran). Hum Ecol Risk Assess 25(4):988–1005. https://doi.org/10.1080/10807039.2018.1458596
Abdalla OAE, Ali M, Al-Higgi K, Al-Zidi H, El-Hussain I, Al-Hinai S (2010) Rate of seawater intrusion estimated by geophysical methods in an arid area. Hydrogeol J 18:1437–1445. https://doi.org/10.1007/s10040-010-0606-0
Adhikary SK, Manjur-A-Elahi M, Hossain AMI (2012) Assessment of shallow groundwater quality from six wards of Khulna City Corporation, Bangladesh. Int J Res Appl Sci Eng 1:488–498. https://doi.org/10.6088/ijaser.0020101050
Akter T, Jhohura FT, Akter F, Chowdhury TR, Mistry SK, Dey D, Barua MK, Islam MA, Rahman M (2016) Water Quality Index for measuring drinking water quality in rural Bangladesh: a cross-sectional study. J Health Popul Nutr 35:4. https://doi.org/10.1186/s41043-016-0041-5
Basavaraddi SB, Kousar H, Puttaiah ET (2012) Dissolved oxygen concentration-a remarkable indicator of groundwater pollution in and around Tiptur town, Tumkur District, Karnataka, India. Bull Environ Pharm Life Sci 1:48–54
Batabyal AK, Chakraborty S (2015) Hydrogeochemistry and water quality index in the assessment of groundwater quality for drinking use. Water Environ Res 87:607–617. https://doi.org/10.2175/106143015X14212658613956
BIS (1991) Bureau of Indian Standards. Indian Standard Drinking Water-Specification. 1st rev. Bureau of Indian Standards: New Dehli, India
Cataldo DA, Aarón M, Schrader LE, Youngs VL (1975) Rapid colourimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun Soil Sci Plan 6:71–80
Collin ML, Melloul AJ (2003) Assessing groundwater vulnerability to pollution to promote sustainable urban and rural development. J Clean Prod 11:727–736. https://doi.org/10.1016/S0959-6526(02)00131-2
DPHE (2018) Department of Public Health Engineering, Government of Bangladesh. http://old.dphe.gov.bd/index.php?option¼com_content&view¼article&id¼125&Itemid¼133. Accessed 10 Feb 2020
ECR (1997) Environmental Conservation Rules of Bangladesh. Ministry of Environment and Forest, Government of Bangladesh
Foster SSD, Chilton PJ (2003) Groundwater: the processes and global significance of aquifer degradation. Philos Trans R Soc Lond B Biol Sci 358:1957–1972. https://doi.org/10.1098/rstb.2003.1380
Gupta M, Srivastava PK (2010) Integrating GIS and remote sensing for identification of groundwater potential zones in the hilly terrain of Pavagarh, Gujarat, India. Water Int 35:233–245. https://doi.org/10.1080/02508061003664419
Harris DC (2010) Quantitative chemical analysis. Macmillan
Hasan MK, Shahriar A, Jim KU (2019) Water pollution in Bangladesh and its impact on public health. Heliyon 5:e02145. https://doi.org/10.1016/j.heliyon.2019.e02145
Hoque M, Hasan MK, Ravenscroft P (2003) Investigation of groundwater salinity and gas problems in south-east Bangladesh. In: Rahman AA, Ravenscroft P (eds) Groundwater resources and development in Bangladesh. Bangladesh Centre for Advanced Studies (BCAS), University Press Ltd, Dhaka
Horton RK (1965) An index number system for rating water quality. J Water Pollut Control Fed 37(3):300–306
Howladar MF, Al Numanbakth MA, Faruque MO (2018) An application of Water Quality Index (WQI) and multivariate statistics to evaluate the water quality around Maddhapara Granite Mining Industrial Area, Dinajpur, Bangladesh. Environ Syst Res 6:13. https://doi.org/10.1186/s40068-017-0090-9
Islam SDU, Bhuiyan MAH, Rume T, Azam G (2017) Hydrogeochemical investigation of groundwater in the shallow coastal aquifer of Khulna District, Bangladesh. Appl Water Sci 7:4219–4236. https://doi.org/10.1007/s13201-017-0533-5
IWM (2011) Institute of Water Modelling, Bangladesh: Khulna Water Supply Project, Government of the People’s Republic of Bangladesh, The Asian Development Bank TA 7385-BAN. Project Number: P42171-012
Karro E, Marandi A, Vaikm R (2004) The origin of increased salinity in the Cambrian-Vendian aquifer system on the Kopl Peninsula, northern Estonia. Hydrogeol J 12:424–435. https://doi.org/10.1007/s10040-004-0339-z
Kim RH, Kim JH, Ryu JS, Chang HW (2006) Salinization properties of shallow groundwater in a coastal reclaimed area, Yeonggwang, Korea. Environ Geol 49:1180–1194. https://doi.org/10.1007/s00254-005-0163-3
Krishnaraj S, Kumar S, Elango KP (2015) Spatial analysis of groundwater quality using geographic information system—a case study. IOSR J Environ Sci Toxicol Food Technol 9:1–6
Kumar SK, Bharani R, Magesh NS, Godson PS, Chandrasekar N (2014) Hydrogeochemistry and groundwater quality appraisal of part of south Chennai coastal aquifers, Tamil Nadu, India using WQI and fuzzy logic method. Appl Water Sci 4:341–350. https://doi.org/10.1007/s13201-013-0148-4
Logeshkumaran A, Magesh N, Godson PS, Chandrasekar N (2015) Hydro-geochemistry and application of water quality index (WQI) for groundwater quality assessment, Anna Nagar, part of Chennai City, Tamil Nadu, India. Appl Water Sci 5:335–343. https://doi.org/10.1007/s13201-014-0196-4
Mekonnen MM, Hoekstra AY (2016) Four billion people facing severe water scarcity. Sci Adv 2:e1500323. https://doi.org/10.1126/sciadv.1500323
Milovanovic M (2007) Water quality assessment and determination of pollution sources along the Axios/Vardar River, Southeastern Europe. Desalination 213(1–3):159–173. https://doi.org/10.1016/j.desal.2006.06.022
Mohr KF (1856) Lehrbuch der chemisch-analytischen Titrirmethode. Vieweg, Braunschweig
Murugesan A, Ramu A, Kannan N (2006) Water quality assessment for Uttamapalayan Municipality in Theni District, Tamil Nadu, India. Pollut Res 25:163–166
NDWQS (2004) National Drinking Water Quality Standard, 2nd edn. Engineering of Services Division, Ministry of Health Malaysia
Nickson RT, McArthur JM, Shrestha B, Kyaw-Nyint TO, Lowry D (2005) Arsenic and other drinking water quality issues, Muzaffargarh District, Pakistan. Appl Geochem 20:55–68. https://doi.org/10.1016/j.apgeochem.2004.06.004
Ramakrishnaiah CR, Sadashivaiah C, Ranganna G (2009) Assessment of water quality index for the groundwater in Tumkur Taluk, Karnataka state, India. E-J Chem 6:523–530. https://doi.org/10.1155/2009/757424
Reza R, Singh G (2010) Assessment of groundwater quality status by using water quality index method in Orissa, India. World Appl Sci J 9:1392–1397
Roy MK, Datta DK, Adhikari DK, Chowdhury BK, Roy PJ (2005) Geology of the Khulna city corporation. J Life Earth Sci 1:57–63
Saeedi M, Abessi O, Sharifi F, Meraji H (2010) Development of groundwater quality index. Environ Monit Assess 163:327–335. https://doi.org/10.1007/s10661-009-0837-5
Sawyer CN, Perry LM, Gene FP (2003) Chemistry for environmental engineering and science, 5th edn. McGraw-Hill, Boston
Sikder MT, Mistri NA, Rahaman MS, Saiara N (2014) Evaluation of groundwater quality and environmental health in the coastal belt of Khulna, Bangladesh. J Bio Pharm Chem Res 1:113–122
Sinha DK, Shilpi S, Ritesh S (2004) Water quality index for Ram Ganga river at Mordabad. Poll Res 23(3):527–531
Stambuk-Giljanovic N (1999) Water quality evaluation by index in dalmatia. Water Res 33(16):3423–3440. https://doi.org/10.1016/S0043-1354(99)00063-9
Tiwari AK, Singh PK, Mahato MK (2014) GIS-based evaluation of water quality index of groundwater resources in West Bokaro Coalfield, India. Curr World Environ 9:843–850. https://doi.org/10.12944/CWE.9.3.35
Van Camp M, Mtoni YE, Mjemah IC, Bakundukize C, Walraevens K (2014) Investigating seawater intrusion due to groundwater pumping with schematic model simulations: the example of the Dar Es Salaam coastal aquifer in Tanzania. J Afr Earth Sci 96:71–78. https://doi.org/10.1016/j.jafrearsci.2014.02.012
Vasanthavigar M, Srinivasamoorthy K, Vijayaragavan K, Ganthi RR, Chidambaram S, Anandhan P, Manivannan R, Vasudevan S (2010) Application of water quality index for groundwater quality assessment: thirumanimuttar sub-basin, Tamilnadu, India. Environ Monit Assess 171:595–609. https://doi.org/10.1007/s10661-009-1302-1
WHO (2006) World Health Organization. Guidelines for drinking water quality, Geneva
WHO (2011) Guidelines for drinking-water quality. In: Health criteria and other supporting information, 4th edn. WHO, Geneva
Wikipedia (2020) Khulna. https://en.wikipedia.org/wiki/Khulna. Accessed 26 Feb 2020
Woobaidullah ASM, Hasan MA, Reza MH, Noor A, Amin MK (2006) Groundwater potentiality-a review of the hydrogeological data available in the coastal belt of Khulna and Satkhira districts. Dhaka Univ J Sci 42:229–233
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahmud, A., Sikder, S. & Joardar, J.C. Assessment of groundwater quality in Khulna city of Bangladesh in terms of water quality index for drinking purpose. Appl Water Sci 10, 226 (2020). https://doi.org/10.1007/s13201-020-01314-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-020-01314-z