Zebrafish as a Successful Animal Model for Screening Toxicity of Medicinal Plants
Abstract
:1. Introduction
2. Zebrafish as a Suitable Alternative Animal Model for Toxicity Tests
- A wide range of chemicals may be relevant;
- Embryos are short-lived, susceptible, cost-effective, and have low variability;
- The regulatory and scientific communities consider them to be standardized;
- The tolerance of various species or organisms can be compared [58].
3. The Most Relevant Behavioral Effects and Basic Methodology Based on Toxicological Assessment Observed in Zebrafish Larvae
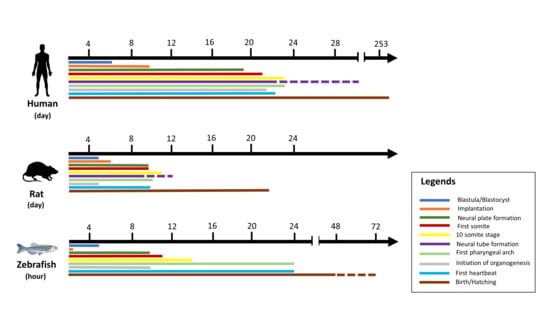
4. Using Zebrafish Embryos to Detect Developmental Toxicity
5. Defects in Zebrafish Organs Found in Developmental Toxicity Studies
5.1. Heart (Cardiotoxicity)
5.2. Gills
5.3. Tail
5.4. Yolk Sac
5.5. Hatchability
6. Chemical Studies of Embryotoxicity
6.1. Salinity
6.2. Phytochemical Compounds
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alafiatayo, A.A.; Lai, K.S.; Syahida, A.; Mahmood, M.; Shaharuddin, N.A. Phytochemical evaluation, embryotoxicity, and teratogenic effects of Curcuma longa extract on zebrafish (Danio rerio). Evid. Based Complement. Altern. Med. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Ismail, H.F.; Hashim, Z.; Soon, W.T.; Ab Rahman, N.S.; Zainudin, A.N.; Abdul Majid, F.A. Comparative study of herbal plants on the phenolic and flavonoid content, antioxidant activities and toxicity on cells and zebrafish embryo. J. Tradit. Complement. Med. 2017, 7, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Falcao, M.A.P.; de Souza, L.S.; Dolabella, S.S.; Guimaraes, A.G.; Walker, C.I.B. Zebrafish as an alternative method for determining the embryo toxicity of plant products: A systematic review. Environ. Sci. Pollut. Res. Int. 2018, 25, 35015–35026. [Google Scholar] [CrossRef]
- Gebrelibanos Hiben, M.; Kamelia, L.; de Haan, L.; Spenkelink, B.; Wesseling, S.; Vervoort, J.; Rietjens, I.M.C.M. Hazard assessment of Maerua subcordata (Gilg) DeWolf. for selected endpoints using a battery of in vitro tests. J. Ethnopharmacol. 2019, 241, 111978. [Google Scholar]
- Fürst, R.; Zündorf, I. Evidence-based phytotherapy in Europe: Where do we stand? Planta Med. 2015, 81, 962–967. [Google Scholar] [CrossRef]
- Yumnamcha, T.; Roy, D.; Devi, M.D.; Nongthoma, U. Evaluation of developmental toxicity and apoptotic induction of the aqueous extract of Millettia pachycarpa using zebrafish as model organism. Toxicol. Environ. Chem. 2015, 97, 1363–1381. [Google Scholar] [CrossRef]
- Little, J.G.; Marsman, D.S.; Baker, T.R.; Mahony, C. In silico approach to safety of botanical dietary supplement ingredients utilizing constituent-level characterization. Food Chem. Toxicol. 2017, 107, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, M.; Gong, Z.; Zonyane, S.; Xu, S.; Makunga, N.P. Comparative cardio and developmental toxicity induced by the popular medicinal extract of Sutherlandia frutescens (L.) R.Br. detected using a zebrafish Tuebingen embryo model. BMC Complement. Altern. Med. 2018, 18, 273. [Google Scholar]
- Mclaughlin, J.L.; Chang, C.J.; Smith, D.L. Bench Top Bioassays for the Discovery of Bioactive Natural Products: An Update. In Studies in Natural Products Chemistry; Atta-Ur-Rahaman, Ed.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 9, pp. 383–409. [Google Scholar]
- Yumnamcha, T.; Nongthomba, U.; Devi, M.D. Phytochemical screening and evaluation of genotoxicity and acute toxicity of aqueous extract of Croton tiglium L. Int. J. Sci. Res. Publ. 2014, 4, 1–5. [Google Scholar]
- Caballero, M.V.; Candiracci, M. Zebrafish as Toxicological model for screening and recapitulate human diseases. J. Unexplor. Med. Data 2018, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Thiagarajan, S.K.; Krishnan, K.R.; Ei, T.; Shafie, N.H.; Arapoc, D.J.; Bahari, H. Evaluation of the effect of aqueous Momordica charantia Linn. extract on zebrafish embryo model through acute toxicity assay assessment. Evid. Based Complement. Alternat. Med. 2019, 9152757. [Google Scholar] [CrossRef] [Green Version]
- Bambino, K.; Chu, J. Zebrafish in toxicology and environmental health. Curr. Top. Dev. Biol. 2017, 124, 331–367. [Google Scholar]
- Heideman, W.; Antkiewicz, D.S.; Carney, S.A.; Peterson, R.E. Zebrafish and cardiac toxicology. Cardiovasc. Toxicol. 2005, 5, 203–214. [Google Scholar] [CrossRef]
- Ayub, A.D.; Hock, I.C.; Mat Yusuf, S.N.A.; Abd Kadir, E.; Ngalim, S.H.; Lim, V. Biocompatible disulphide cross-linked sodium alginate derivative nanoparticles for oral colon-targeted drug delivery. Artif. Cells Nanomed. Biotechnol. 2019, 47, 353–369. [Google Scholar] [CrossRef] [Green Version]
- Abuchenari, A.; Hardani, K.; Abazari, S.; Naghdi, F.; Ahmady Keleshteri, M.; Jamavari, A.; Modarresi Chahardehi, A. Clay-reinforced nanocomposites for the slow release of chemical fertilizers and water retention. J. Compos. Comp. 2020, 2, 85–91. [Google Scholar] [CrossRef]
- Nishimura, Y.; Inoue, A.; Sasagawa, S.; Koiwa, J.; Kawaguchi, K.; Kawase, R.; Maruyama, T.; Kim, S.; Tanaka, T. Using zebrafish in systems toxicology for developmental toxicity testing. Congenit. Anom. Kyoto 2016, 56, 18–27. [Google Scholar]
- Han, H.S.; Jang, G.H.; Jun, I.; Seo, H.; Park, J.; Glyn-Jones, S.; Seok, H.K.; Lee, K.H.; Montovani, D.; Kim, Y.C.; et al. Transgenic zebrafish model for quantification and visualization of tissue toxicity caused by alloying elements in newly developed biodegradable metal. Sci. Rep. 2018, 8, 13818. [Google Scholar] [CrossRef]
- Rennekamp, A.J.; Peterson, R.T. 15 years of zebrafish chemical screening. Curr. Opin. Chem. Biol. 2015, 24, 58–70. [Google Scholar] [CrossRef] [Green Version]
- Van der Laan, J.W.; Chapin, R.E.; Haenen, B.; Jacobs, A.C.; Piersma, A. Testing strategies for embryo-fetal toxicity of human pharmaceuticals. Animal models vs. in vitro approaches: A workshop report. Regul. Toxicol. Pharmacol. 2012, 63, 115–123. [Google Scholar] [CrossRef]
- Cassar, S.; Adatto, I.; Freeman, J.L.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; Van Cruchten, S.; Zon, L.I. Use of zebrafish in drug discovery toxicology. Chem. Res. Toxicol. 2020, 33, 95–118. [Google Scholar] [CrossRef] [Green Version]
- Jayasinghe, C.D.; Jayawardena, U.A. Toxicity Assessment of Herbal Medicine Using Zebrafish Embryos: A Systematic Review. Evid. Based Complement. Altern. Med. 2019, 7272808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizgirev, I.V.; Revskoy, S. A new zebrafish model for experimental leukemia therapy. Cancer Biol. Ther. 2010, 9, 895–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, D.H.; Roo, B.D.; Nguyen, X.B.; Vervaele, M.; Kecskes, A.; Ny, A.; Copmans, D.; Vriens, H.; Locquet, J.P.; Hoet, P.; et al. Use of zebrafish larvae as a multi-endpoint platform to characterize the toxicity profile of Silica Nanoparticles. Sci. Rep. 2016, 6, 37145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caballero, M.V.; Candiracci, M. Zebrafish as screening model for detecting toxicity and drugs efficacy. J. Unexplor. Med. Data 2018, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Garcia, G.R.; Noyes, P.D.; Tanguay, R.L. Advancements in zebrafish applications for 21st century toxicology. Pharmacol. Ther. 2016, 161, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermsen, S.A.B.; van den Brandhof, E.J.; van der Ven, L.T.M.; Piersma, A.H. Relative embryotoxicity of two classes of chemicals in a modified zebrafish embryotoxicity test and comparison with their in vivo potencies. Toxicol. In Vitro 2011, 25, 745–753. [Google Scholar] [CrossRef] [Green Version]
- Fazry, S.; Mohd Noordin, M.A.; Sanusi, S.; Mat Noor, M.; Aizat, W.M.; Mat Lazim, A.; Dyari, H.R.E.; Jamar, N.H.; Remali, J.; Othman, B.A.; et al. Cytotoxicity and toxicity evaluation of Xanthone crude extract on hypoxic human hepatocellular carcinoma and zebrafish (Danio rerio) embryos. Toxics 2018, 6, 60. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, R. Zebrafish Early Life-stages and Adults as A Tool for Ecotoxicity Assessment. Master’s Thesis, Universidade de Aveiro, Aveiro, Portugal, 2009. [Google Scholar]
- Rizzo, L.Y.; Golombek, S.K.; Mertens, M.E.; Pan, Y.; Laaf, D.; Broda, J.; Jayapaul, J.; Mockel, D.; Subr, V.; Hennink, W.E.; et al. In vivo nanotoxicity testing using the zebrafish embryo assay. J. Mater. Chem. B. 2013, 1, 3918–3925. [Google Scholar] [CrossRef]
- D’Amora, M.; Giordani, S. The Utility of Zebrafish as a Model for Screening Developmental Neurotoxicity. Front. Neurosci. 2018, 12, 976. [Google Scholar] [CrossRef] [Green Version]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Koziol, E.; Deniz, F.S.S.; Orhan, I.E.; Marcourt, L.; Budzynska, B.; Wolfender, J.L.; Crawford, A.D.; Skalicka-Wozniak, K. High-performance counter-current chromatography isolation and initial neuroactivity characterization of furanocoumarin derivatives from Peucedanum alsaticum L (Apiaceae). Phytomed 2019, 54, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.S. Zebrafish: A complete animal model to enumerate the nanoparticle toxicity. J. Nanobiotechnol. 2016, 14, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakaria, Z.Z.; Benslimane, F.M.; Nasrallah, G.K.; Shurbaji, S.; Younes, N.N.; Mraiche, F.; Da’as, S.I.; Yalcin, H.C. Using zebrafish for investigating the molecular mechanisms of drug-induced cardiotoxicity. BioMed Res. Int. 2018, 2018, 1642684. [Google Scholar] [CrossRef] [PubMed]
- Sant, K.E.; Timme-Laragy, A.R. Zebrafish as a model for toxicological perturbation of yolk and nutrition in the early embryo. Curr. Environ. Health Rep. 2018, 5, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Lammer, E.; Wendler, K.; Rawling, J.M.; Belanger, S.E.; Braunbeck, T. Is the fish embryo toxicity test (FET) with the zebrafish (Danio rerio) a potential alternative for the fish acute toxicity test? Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2009, 149, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Oliveri, A.; Levin, E.D. Zebrafish model systems for developmental neurobehavioral toxicology. Birth Defects Res. Part C Embryo Today Rev. 2013, 99, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Li, C.; Wang, C.; Peng, Y.; Shu, L.; Jia, X.; Ma, W.; Wang, B. Metabolism of tanshinone IIA, cryptotanshinone and tanshinone I from Radix Salvia miltiorrhiza in zebrafish. Molecules 2012, 17, 8617–8632. [Google Scholar] [CrossRef] [Green Version]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ulimann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Carlson, B.M. Human Embryology and Developmental biology E-book; Elsevier Health Sciences: St. Louis, MI, USA, 2018. [Google Scholar]
- Ali, S.; van Mil, H.G.; Richardson, M.K. Large-scale assessment of the zebrafish embryo as a possible predictive model in toxicity testing. PLoS ONE 2011, 6, e21076. [Google Scholar] [CrossRef] [Green Version]
- Syahbirin, G.; Mumuh, N.; Mohamad, K. Curcuminoid and toxicity levels of ethanol extract of Javanese ginger (Curcuma xanthorriza) on brine shrimp (Artemia salina) larvae and zebrafish (Danio rerio) embryos. Asian J. Pharm. Clin. Res. 2017, 10, 169–173. [Google Scholar] [CrossRef] [Green Version]
- Littleton, R.M.; Hove, J.R. Zebrafish: A nontraditional model of traditional medicine. J. Ethnopharmacol. 2013, 145, 677–685. [Google Scholar]
- Wang, T.; Wang, C.; Wu, Q.; Zheng, K.; Chen, J.; Lan, Y.; Qin, Y.; Mei, W.; Wang, B. Evaluation of tanshinone IIA developmental toxicity in zebrafish embryos. Molecules 2017, 22, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braunbeck, T.; Boettcher, M.; Hollert, H.; Kosmehl, T.; Leist, E.; Rudolf, M.; Seitz, N. Towards an alternative for the acute fish LC50 test in chemical assessment: The fish embryo toxicity test goes multi-species -- an update. ALTEX 2005, 22, 87–102. [Google Scholar] [PubMed]
- Yang, L.; Ho, N.Y.; Alshut, R.; Legradi, J.; Weiss, C.; Reischl, M.; Mikut, R.; Liebel, U.; Muller, F.; Strahle, U. Zebrafish embryos as models for embryotoxic and teratological effects of chemicals. Reprod. Toxicol. 2009, 28, 245–253. [Google Scholar] [CrossRef]
- Di Paolo, C.; Seiler, T.B.; Keiter, S.; Hu, M.; Muz, M.; Brack, W.; Hollert, H. The value of zebrafish as an integrative model in effect-directed analysis—A review. Environ. Sci. Eur. 2015, 27, 8. [Google Scholar] [CrossRef] [Green Version]
- Stern, H.M.; Zon, L.I. Cancer genetics and drug discovery in the zebrafish. Nat. Rev. Cancer 2003, 3, 533–539. [Google Scholar] [CrossRef]
- R, E.B.; Jesubatham, P.D.; Berlin Grace, V.M.; Viswanathan, S.; Srividya, S. Non-toxic and non teratogenic extract of Thuja orientalis L. inhibited angiogenesis in zebra fish and suppressed the growth of human lung cancer cell line. Biomed. Pharmacother. 2018, 106, 699–706. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Gallagher, E.; O’Connor, P.; Prieto, J.; Mora-soler, L.; Grealy, M.; Hayes, M. Development of a seaweed derived platelet activating factor acetylhydrolase (PAF-AH) inhibitory hydrolysate, synthesis of inhibitory peptides and assessment of their toxicity using the zebrafish larvae assay. Peptides 2013, 50, 119–124. [Google Scholar] [CrossRef]
- Bertelli, P.R.; Biegelmeyer, R.; Rico, E.P.; Klein-Junior, L.C.; Toson, N.S.B.; Minetto, L.; Bordignon, S.A.L.; Gasper, A.L.; Moura, S.; de Oliveira, D.L.; et al. Toxicological profile and acetylcholinesterase inhibitory potential of Palicourea deflexa, a source of beta-carboline alkaloids. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017, 201, 44–50. [Google Scholar] [CrossRef]
- Tolentino, J.; Undan, J.R. Embryo-toxicity and teratogenicity of Derris elliptica leaf extract on zebraish (Danio rerio) embryos. Int. J. Pure Appl. Biosci. 2016, 4, 16–20. [Google Scholar] [CrossRef]
- He, J.H.; Gao, J.M.; Huang, C.J.; Li, C.Q. Zebrafish models for assessing developmental and reproductive toxicity. Neurotoxicol. Teratol. 2014, 42, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Yu, L.; Liu, C.; Yu, K.; Shi, X.; Yeung, L.W.Y.; Lam, P.K.S.; Wu, R.S.S.; Zhou, B. Hexabromocyclododecane-induced developmental toxicity and apoptosis in zebrafish embryos. Aquat. Toxicol. 2009, 93, 29–36. [Google Scholar] [CrossRef]
- Carwan, M.J., III; Heiden, T.K.; Tomasiewicz, H.C. The utility of zebrafish as a model for toxicological research. In Biochemistry and Molecular Biology of Fishes; Mommsen, T.P., Moon, T.W., Eds.; Chapter 1; Elsevier: New York, NY, USA, 2005; Volume 6, pp. 3–41. [Google Scholar]
- Knobel, M.; Busser, F.J.M.; Rico-Rico, A.; Kramer, N.I.; Hermens, J.L.M.; Hafner, C.; Tanneberger, K.; Schirmer, K.; Scholz, S. Predicting adult fish acute lethality with the zebrafish embryo: Relevance of test duration, endpoints, compound properties, and exposure concentration analysis. Environ. Sci. Technol. 2012, 46, 9690–9700. [Google Scholar] [CrossRef] [PubMed]
- Fairbrother, A.; Lewis, M.A.; Menzer, R.E. Methods in environmental toxicology. In Principles and Methods of Toxicology, 4th ed.; Hayes, A.W., Ed.; Chapter 42; CRC Press, Taylor & Francis Group: London, UK, 2001; pp. 1759–1801. [Google Scholar]
- Massei, R.; Hollert, H.; Krauss, M.; von Tumpling, W.; Weidauer, C.; Haglund, P.; Kuster, E.; Gallampois, C.; Tysklind, M.; Brack, W. Toxicity and neurotoxicity profiling of contaminated sediments from Gulf of Bothnia (Sweden): A multi-endpoint assay with Zebrafish embryos. Environ. Sci. Eur. 2019, 31, 8. [Google Scholar] [CrossRef] [Green Version]
- Nagel, R. DarT: The embryo test with the zebrafish Danio rerio--a general model in ecotoxicology and toxicology. ALTEX 2002, 19, 38–48. [Google Scholar] [PubMed]
- Liedtke, A.; Muncke, J.; Rufenacht, K.; Eggen, R.I.L. Molecular multi-effect screening of environmental pollutants using the MolDarT. Environ. Toxicol. 2008, 23, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Njiwa, J.R.K.; Suter, M.J.-F.; Eggen, R.I. Zebrafish Embryo Toxicity Assay, Combining Molecular and Integrative Endpoints at Various Developmental Stages. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 4481–4489. [Google Scholar]
- Bugel, S.M.; Bonventre, J.A.; Tanguay, R.L. Comparative developmenal toxicity of flavonoids using an integrative zebrafish system. Toxicol. Sci. 2016, 154, 55–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haldi, M.; HArden, M.; D’Amico, L.; DeLise, A.; Seng, W.L. Developmental toxicity assessment in zebrafish. In Zebrafish: Methods for Assessing Drug Safety and Toxicity; McGrath, P., Ed.; Phylonix: Cambridge, MA, USA, 2011. [Google Scholar]
- Busquet, F.; Strecker, R.; Rawlings, J.M.; Belnger, S.E.; Braunbeck, T.; Carr, G.J.; Cenijn, P.; Fochtman, P.; Gourmelon, A.; Hubler, N.; et al. OECD validation study to assess intra and inter-laboratory reproducibility of the zebrafish embryo toxicity test for acute aquatic toxicity testing. Regul. Toxicol. Pharmacol. 2014, 69, 496–511. [Google Scholar] [CrossRef]
- Parng, C.; Seng, W.L.; Semino, C.; McGrath, P. Zebrafish: A preclinical model for drug screening. Assay Drug Dev. Technol. 2002, 1, 41–48. [Google Scholar] [CrossRef]
- OECD. Fish Embryo Toxicity Test; Organization for Economic Cooperation and Development: Paris, France, 2006. [Google Scholar]
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawia, J.S.; Craddock, C.; Kyzar, E.; Roth, A.; Landsman, S.; et al. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef]
- Chueh, T.C.; Hsu, L.S.; Kao, C.M.; Hsu, T.W.; Liao, H.Y.; Wang, K.Y.; Chen, S.C. Transcriptome analysis of zebrafish embryos exposed to deltamethrin. Environ. Toxicol. 2017, 32, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Han, Y.; Liu, Y.; Zhang, J.; Hu, C. Relationship between fluoroquinolone structure and neurotoxicity revealed by zebrafish neurobehavior. Chem. Res. Toxicol. 2018, 31, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.Y.; Cowden, J.; Simmons, S.O.; Padilla, S.; Ramabhadran, R. Gene expression changes in developing zebrafish as potential markers for rapid developmental neurotoxicity screening. Neurotoxicol. Teratol. 2009, 32, 91–98. [Google Scholar] [CrossRef] [PubMed]
- OECD. Fish Acute Toxicity Test; Organization for Economic Cooperation and Development: Medmenham, UK, 1992. [Google Scholar]
- Shaikh, A.; Kohale, K.; Ibrahim, M.; Khan, M. Teratogenic effects of aqueous extract of Ficus glomerata leaf during embryonic development in zebrafish (Danio rerio). J. Appl. Pharm. Sci. 2019, 9, 107–111. [Google Scholar]
- Paramanik, V.; Basumatary, M.J.; Nagesh, G.; Kushwaha, J.K.; Kurrey, K. Ethanolic extracts of candidate Indian Traditional Medicines Acorus calamus, Terminalia chebula and Achyranthes aspera are neuroprotective in Zebrafish. IBRO Rep. 2019, 6, S247. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, S.; Wang, C.; Zhang, Q. A fructooligosaccharide from Achyranthes bidentata inhibits osteoporosis by stimulating bone formation. Carbohydr. Polym. 2019, 210, 110–118. [Google Scholar] [CrossRef]
- Aleksandar, P.; Dragana, M.C.; Nebojsa, J.; Biljana, N.; Natasa, S.; Branka, V.; Jelena, K.V. Wild edible onions—Allium flavum and Allium carinatum—Successfully prevent adverse effects of chemotherapeutic drug doxorubicin. Biomed. Pharmacother. 2019, 109, 2482–2491. [Google Scholar] [CrossRef]
- Ponrasu, T.; Ganeshkumar, M.; Suguna, L. Developmental toxicity evaluation of ethanolic extract of Annona squamosa in zebrafish (Danio rerio) embryo. J. Pharm. Res. 2012, 5, 277–279. [Google Scholar]
- Grisola, M.A.C.; Fuentes, R.G. Phenotype-based screening of selected mangrove methanolic crude extracts with anti-melanogenic activity using zebrafish (Danio rerio) as a model. ScienceAsia 2017, 43, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Batista, F.L.A.; Lima, L.M.G.; Abrante, I.A.; de Araujo, J.I.F.; Batista, F.L.A.; Abrante, I.A.; Lima, M.C.L.; do Prado, B.S.; Moura, L.F.W.G.; Guedes, M.I.F.; et al. Antinociceptive activity of ethanolic extract of Azadirachta indica A. Juss (Neem, Meliaceae) fruit through opioid, glutamatergic and acid-sensitive ion pathways in adult zebrafish (Danio rerio). Biomed. Pharmacother. 2018, 108, 408–416. [Google Scholar]
- Teh, L.E.; Kue, C.S.; Ng, C.H.; Lau, B.F. Toxicity effect of Bougainvillea glabra (paper flower) water extracts on zebrafish embryo. INNOSC Ther. Pharmacol. Sci. 2019, 2, 23–26. [Google Scholar] [CrossRef] [Green Version]
- De Vera, J.S.; De Castro, M.E.G.; Dulay, R.M.R. Phytochemical constituents and teratogenic effect of lyophilized extracts of Bixa orellana L. (Achuete) and Piper betle L. (Ikmo) leaves in Danio rerio embryos. Der Pharma Chem. 2016, 8, 432–437. [Google Scholar]
- Vargas-Mendez, L.Y.; Rosado-Solano, D.N.; Sanabria-Florez, P.L.; Puerto-Galvis, C.E.; Kouznetsov, V. In vitro antioxidant and anticholinesterase activities and in vivo toxicological assessment (Zebrafish embryo model) of ethanolic extracts of Capsicum chinense Jacq. J. Med. Plants Res. 2016, 10, 59–66. [Google Scholar]
- Xia, Q.; Ma, Z.; Luo, J.; Wang, Y.; Li, T.; Feng, Y.; Ni, Y.; Zou, Q.; Lin, R. Assay for the developmental toxicity of safflower (Carthamus tinctorius L.) to zebrafish embryos/larvae. J. Tradit. Chin. Med. Sci. 2017, 4, 71–81. [Google Scholar]
- Murugesu, S.; Khatib, A.; Ahmad, Q.U.; Ibrahim, Z.; Uzir, B.F.; Benchoula, K.; Yusoff, N.I.N.; Perumal, V.; Aljami, M.F.; Salamah, S.; et al. Toxicity study on Clinacanthus nutans leaf hexane fraction using Danio rerio embryos. Toxicol. Rep. 2019, 6, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Orellana-Paucar, A.M.; Serruys, A.S.K.; Afrikanova, T.; Maes, J.; De Borggraeve, W.; Alen, J.; Leon-Tamariz, F.; Wilches-Arizabala, I.M.; Crawford, A.M.; de Witte, P.A.M.; et al. Anticonvulsant activity of bisabolene sesquiterpenoids of Curcuma longa. Epilepsy Behav. 2012, 24, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.R.; Vincent, S.G. Cynodon dactylon and Sida acuta extracts impact on the function of the cardiovascular system in zebrafish embryos. J. Biomed. Res. 2012, 26, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Memita, A.Y.B.; Macalinao, S.D.; Reyes, L.E.G.; Damian, E.E.; Dulay, R.M.R. Toxicity and teratogenicity of Dieffenbachia amoena leaf extract. Int. J. Biol. Pharm. Allied Sci. 2018, 7, 1591–1600. [Google Scholar]
- Flaviano, J.F.; Kenniker, M.A.A.; Ganda, F.J.C.; Sacedor, J.; Lee, M.Y.S.; Cabuhat, K.S.P. Assessment of the toxic and teratogenic effects of Kamagong (Diospyros discolor Willd) leaves extracts to the developing embryo of zebrafish (Danio rerio). Egypt. Acad. J. Biol. Sci. B. Zool. 2018, 10, 53–60. [Google Scholar] [CrossRef]
- Ponpornpisit, A.; Pirarat, N.; Suthikrai, W.; Binwihok, A. Toxicity test of Kameng (Ecliptra prostrate Linn.) and Kradhuawean (Spilanthes acmella (Linn.) Murr.) to early life stage of zebrafish (Danio rerio). Thai. J. Vet. Med. 2011, 41, 523–527. [Google Scholar]
- Xavier, J.; Kripasana, K. Acute Toxicity of Leaf Extracts of Enydra fluctuans Lour in Zebrafish (Danio rerio Hamilton). Sci. Cairo 2020, 2020, 3965376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, B.; Feng, Y.; Liu, J.; Ma, Z.; Zheng, J.; Xia, Q.; Ni, Y.; Li, F.; Lin, R. Anti-angiogenic activity of water extract from Euphorbia pekinensis Rupr. J. Ethnopharmacol. 2017, 206, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ma, L.; Li, S.; Cui, K.; Lei, L.; Ye, Z. Evaluation of the cardiotoxicity of evodiamine in vitro and in vivo. Molecules 2017, 22, 943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.L.; Li, K.; Lin, Q.H.; Ren, J.; He, Z.H.; Li, H.; Shen, N.; Wei, P.; Feng, F.; He, M.F. Gambogic acid causes fin developmental defect in zebrafish embryo partially via retinoic acid signaling. Reprod. Toxicol. 2016, 63, 161–168. [Google Scholar] [CrossRef]
- Sajkowska-Kozielewicz, J.J.; Kozielewicz, P.; Barnes, N.M.; Wawer, I.; Paradowska, K. Antioxidant, cytotoxic, and antiproliferative activities and total polyphenol contents of the extracts of Geissospermum reticulatum bark. Oxid. Med. Cell. Longev. 2016, 2016, 2573580. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, I.V.F.; de Souza, G.C.; Santana, G.R.; Duarte, J.L.; Fernandes, C.P.; Keia, H.; Velazquez-Moyado, J.A.; Navarrete, A.; Ferreira, I.M.; Carvalho, H.O. Histopathology in zebrafish (Danio rerio) to evaluate the toxicity of medicine: An anti-inflammatory phytomedicine with Janaguba milk (Himatanthus drasticus Plumel). Histopathol. Update 2018, 39–64. [Google Scholar]
- Lira, S.M.; Dionisio, A.P.; Holanda, M.O.; Marques, C.G.; da Silva, G.S.; Correa, L.C.; Santos, G.B.M.; de Abreu, F.A.P.; Magalhaes, F.E.A.; Reboucas, E.D.; et al. Metabolic profile of pitaya (Hylocereus polyrhizus (FA.C. Weber) Britton & Rose) by UPLC-QTOF-MSE and assessment of its toxicity and anxiolytic-like effect in adult zebrafish. Food Res. Intl. 2020, 127, 108701. [Google Scholar]
- He, Y.L.; Shi, J.Y.; Peng, C.; Hu, L.J.; Liu, J.; Zhou, Q.M.; Guo, L.; Xiong, L. Angiogenic effect of motherwort (Leonurus japonicus) alkaloids and toxicity of motherwort essential oil on zebrafish embryos. Fitoterapia 2018, 128, 36–42. [Google Scholar] [CrossRef]
- Wang, W.; Feng, S.; Xiong, Z. Protective effect of polysaccharide from Ligusticum chuanxiong hort against H2O2-induced toxicity in zebrafish embryo. Carbohydr. Polym. 2019, 221, 73–83. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Liu, K.; He, Q.; Sun, C.; Han, J.; Han, L.; Tian, Q. Xiaoaiping induces developmental toxicity in zebrafish embryos through activation of ER stress, apoptosis and the Wnt pathway. Front Pharmacol. 2018, 9, 1250. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.F.; Abutaha, N.; Nasr, F.A.; Alqahtani, A.S.; Noman, O.M.; Wadaan, M.A.M. Bitter gourd (Momordica charantia) possess developmental toxicity as revealed by screening the seeds and fruit extracts in zebrafish embryos. BMC Complement. Altern. Med. 2019, 19, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, M.M.R.; Agpaoa, A.R.; Sayson, A.D.; De Castro, M.E.G.; Dulay, R.M.R. Toxic and teratogenic effects of water leaf extract of Momordica charantia in zebrafish (Danio rerio) embryos. Der Pharma Chem. 2017, 9, 119–122. [Google Scholar]
- David, C.R.S.; Angeles, A.; Angoluan, R.C.; Santos, J.P.E.; David, E.S.; Dulay, R.M.R. Moringa oleifera (Malunggay) water extracts exhibit embryo-toxic and teratogenic activity in zebrafish (Danio rerio) embryo model. Der Pharm. Lett. 2016, 8, 163–168. [Google Scholar]
- Elsayed, E.A.; Farooq, M.; Sharaf-Eldin, M.A.; El-Enshasy, H.A.; Wadaan, M. Evaluation of developmental toxicity and anti-angiogenic potential of essential oils from Moringa oleifera and Moringa peregrina seeds in zebrafish (Danio rerio) model. S. Afr. J. Bot. 2019, 129, 229–237. [Google Scholar] [CrossRef]
- De Santana Silva, L.L.; Alves, R.N.; de Paulo, D.V.; da Silva, J.D.F. Ecotoxicity of water-soluble lectin from Moringa oleifera seeds to zebrafish (Danio rerio) embryos and larvae. Chemosphere 2017, 185, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Babic, S.; Malev, O.; Pflieger, M.; Lebedev, A.T.; Mazur, D.M.; Kuzic, A.; Coz-Rakovac, R.; Trebse, P. Toxicity evaluation of olive oil mill wastewater and its polar fraction using multiple whole-organism bioassays. Sci. Total Environ. 2019, 686, 903–914. [Google Scholar] [CrossRef]
- Safavi, F.; Farimani, M.M.; Golalipour, M.; Leung, P.-C.; Lau, K.-M.; Kwok, H.-F.; Wong, C.-W.; Bayat, H.; Lau, C.B.-S. Investigations on the wound healing properties of Onosma dichroantha Boiss root extracts. S. Afr. J. Bot. 2019, 125, 344–352. [Google Scholar] [CrossRef]
- Santhoshkumar, J.; Sowmya, B.; Kumar, S.V.; Rajeshkumar, S. Toxicology evaluation and antidermatophytic activity of silver nanoparticles synthesized using leaf extract of Passiflora caerulea. S. Afr. J. Chem. Eng. 2019, 29, 17–23. [Google Scholar] [CrossRef]
- Lamban, I.Q.; Balbeuna, E.S.; Lee, M.Y.S.; Sacdalan, M.; Carpio JR, A.P.; Cabuhat, K.S.P. Toxic and teratogenic effects of Sampa-Sampalukan (Phyllanthus niruri) leaves extract using Danio rerio embryo assay. Egypt. Acad. J. Biol. Sci. 2019, 11, 109–115. [Google Scholar] [CrossRef]
- Thanh, D.T.H.; Thanh, N.L.; Thang, N.D. Toxicological and melanin synthesis effects of Polygonum multiflorum root extracts on zebrafish embryos and human melanocytes. Biomed. Res. Ther. 2016, 3, 808–818. [Google Scholar] [CrossRef]
- Yang, J.B.; Li, W.F.; Liu, Y.; Wang, Q.; Cheng, X.L.; Wei, F.; Wang, A.G.; Jin, H.T.; Ma, S.C. Acute toxicity screening of different extractions, components and constituents of Polygonum multiflorum Thunb. on zebrafish (Danio rerio) embryos in vivo. Biomed. Pharmacother. 2018, 99, 205–213. [Google Scholar] [PubMed]
- Xia, Q.; Wei, L.; Zhang, Y.; Kong, H.; Shi, Y.; Wang, X.; Chen, X.; Han, L.; Liu, K. Psoralen induces developmental toxicity in zebrafish embryos/larvae through oxidative stress, apoptosis, and energy metabolism disorder. Front. Pharmacol. 2018, 9, 1457. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, I.; Permadi, K.; Hartati, R.; Damayanti, S. Ethanolic extract of pomegranate (Punica granatum L) peel: Acute toxicity tests on zebrafish (Danio rerio) embryos and its toxicity prediction by in silico. J. Appl. Pharm. Sci. 2018, 8, 082–086. [Google Scholar]
- Huang, Y.; Bai, Y.; Wang, Y.; Kong, H. Solidago canadensis L. extracts to control algal (Microcystis) blooms in ponds. Ecol. Eng. 2014, 70, 263–267. [Google Scholar] [CrossRef]
- Dos Santos Sampaio, T.I.; de Melo, N.C.; de Freitas Paiva, B.T.; da Silva Aleluia, G.A.; da Silva Neto, F.L.P.; da Silva, H.R.; Keita, H.; Cruz, R.A.S.; Sanchez-Ortiz, B.L.; Pineda-Pena, E.A.; et al. Leaves of Spondias mombin L. a traditional anxiolytic and antidepressant: Pharmacological evaluation on zebrafish (Danio rerio). J. Ethnopharmacol. 2018, 224, 563–578. [Google Scholar]
- Kumar, P.B.S.; Kar, B.; Dolai, N.; Haldar, P.K. Study on developmental toxicity and behavioral safety of Streblus asper Lour. bark on zebrafish embryos. Indian J. Nat. Prod. Res. 2013, 4, 255–259. [Google Scholar]
- Zonyane, S.; Chen, L.; Xu, M.J.; Gong, Z.N.; Xu, S.; Makung, N.P. Geographic-based metabolomic variation and toxicity analysis of Sutherlandia frutescens L. R.Br.—An emerging medicinal crop in South Africa. Ind. Crops Prod. 2019, 133, 414–423. [Google Scholar]
- Russo, H.M.; Queiroz, E.F.; Marcourt, L.; Crawford, A.D.; Bolzani, V.; Wolfender, J.L. Chemical profile and toxicity study of Tetrapterys multiglandulosa secondary metabolites using zebrafish toxicity model. In Brazilian Conference on Natural Products and Annual Meeting on Micromolecular Evolution, Systematics and Ecology; GALOÁ: Campinas, Brazil, 2018. [Google Scholar]
- Romagosa, C.M.R.; David, E.S.; Dulay, R.M.R. Embryo-toxic and teratogenic effects of Tinospora cordifolia leaves and bark extracts in Zebrafish (Danio rerio) embryos. Asian J. Plant Sci. Res. 2016, 6, 37–41. [Google Scholar]
- Aleksic, I.; Ristivojevic, P.; Pavic, A.; Radojevic, I.; Comic, L.R.; Vasiljevic, B.; Opsenica, D.; Milojkovic-Opsenica, D.; Senerovic, L. Anti-quorum sensing activity, toxicity in zebrafish (Danio rerio) embryos and phytochemical characterization of Trapa natans leaf extracts. J. Ethnopharmacol. 2018, 222, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.P.; Feng, F.; Zhang, X.Q.; Liu, X.X.; Wang, Y.B.; She, J.X.; He, Z.H.; He, M.F. Toxicity assessment of 7 anticancer compounds in zebrafish. Int. J. Toxicol. 2014, 33, 98–105. [Google Scholar] [CrossRef]
- Puerto Galvis, C.E.; Kouznetsov, V.V. Synthesis of zanthoxylamide protoalkaloids and their in silico ADME-Tox screening and in vivo toxicity assessment in zebrafish embryos. Eur. J. Pharm. Sci. 2019, 127, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Ogungbemi, A.; Leuthold, D.; Scholz, S.; Kuster, E. Hypo- or hyperactivity of zebrafish embryos provoked by neuroactive substances: A review on how experimental parameters impact the predictability of behavior changes. Environ. Sci. Eur. 2019, 31, 88. [Google Scholar] [CrossRef]
- Ma, C.; Parng, C.; Wen, L.S.; Zhang, C.; Willett, C.; McGrath, P. Zebrafish-an in vivo model for drug screening. Innov. Pharm. Technol. 2003, 3, 38–45. [Google Scholar]
- Truong, L.; Harper, S.L.; Tanguay, R.L. Evaluation of embryotoxicity using the zebrafish model. Methods Mol. Biol. 2017, 691, 271–279. [Google Scholar]
- Driever, W.; Stemple, D.; Schier, A.; Solnica-Krezel, L. Zebrafish: Genetic tools for studying vertebrate development. Trends Genet. 1994, 10, 152–159. [Google Scholar] [CrossRef]
- OECD. Guidelines for Testing of Chemical-Fish Embryo Acute Toxicity (FET) Test; OECD Publishing: Paris, France, 2013. [Google Scholar]
- Calienni, M.N.; Feas, D.A.; Igartua, D.E.; Chiaramoni, N.S.; Alonso, S.V.; Prieto, M.J. Nanotoxicological and teratogenic effects: A linkage between dendrimer surface charge and zebrafish developmental stages. Toxicol. Appl. Pharmacol. 2017, 337, 1–11. [Google Scholar] [CrossRef]
- Yamashita, M. Apoptosis in zebrafish development. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 136, 731–742. [Google Scholar] [CrossRef]
- Haffter, P.; Granato, M.; Brand, M.; Mullins, M.C.; Hammerschmidt, M.; Kane, D.A.; Odenthal, J.; van Eeden, F.J.; Jiang, Y.J.; Heisenberg, C.P.; et al. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 1996, 123, 1–36. [Google Scholar]
- Li, H.; Yu, S.; Cao, F.; Wang, C.; Zheng, M.; Li, X.; Qiu, L. Developmental toxicity and potential mechanisms of pyraoxystrobin to zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2018, 151, 1–9. [Google Scholar] [CrossRef]
- Bakkers, J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc. Res. 2011, 91, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Thorarensen, H.; Galiugher, P.E.; Farrel, A.P. The limitations of heart rate as a predictor of metabolic rate in fish. J. Fish Biol. 1996, 49, 226–236. [Google Scholar] [CrossRef]
- Gao, X.Y.; Li, K.; Jiang, L.L.; He, M.F.; Pu, C.H.; Kang, D.; Xie, J. Developmental toxicity of auranofin in zebrafish embryos. J. Appl. Toxicol. 2017, 37, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Liu, H.; Fang, C.; Liu, Y.; Liu, X.; Liu, J. Cardiotoxicity evaluation and comparison of diterpene alkaloids on zebrafish. Drug Chem. Toxicol. 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Farrell, A.P.; Pieperhoff, S. Design and physiology of the heart/cardiac anatomy in fishes. Encycl. Fish Physiol. 2011, 2, 998–1005. [Google Scholar]
- Hu, N.; Sedmera, D.; Yost, H.J.; Clark, E.B. Structure and function of the developing zebrafish heart. Anat. Rec. 2000, 260, 148–157. [Google Scholar] [CrossRef]
- Yamauchi, H.; Hotta, Y.; Konishi, M.; Miyake, A.; Kawahara, A.; Itoh, N. Fgf21 is essential for haematopoiesis in zebrafish. EMBO Rep. 2006, 7, 649–654. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Liu, X.; He, Q.; Chen, X. Toxic effects of celastrol on embryonic development of zebrafish (Danio rerio). Drug Chem. Toxicol. 2011, 34, 61–65. [Google Scholar] [CrossRef]
- Lawson, N.D.; Weinstein, B.M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 2002, 248, 307–318. [Google Scholar] [CrossRef] [Green Version]
- Kinna, G.; Kolle, G.; Carter, A.; Lieschke, G.J.; Perkins, A.; Little, M.H. Knockdown of zebrafish crim1 results in a bent tail phenotype with defects in somite and vascular development. Mech. Dev. 2006, 123, 277–287. [Google Scholar] [CrossRef]
- Cinar, K.; Aksoy, A.; Emre, Y.; Asti, R.N. The histology and histochemical aspects of gills of the flower fish, Pseudophoxinus antalyae. Vet. Res. Commun. 2009, 33, 453. [Google Scholar] [CrossRef]
- Mauceri, A.; Fossi, M.C.; Leonzio, C.; Ancora, S.; Minniti, F.; Maisano, M.; Ferrando, S.; Fasulo, S. Stress factors in the gills of Liza aurata (Perciformes, Mugilidae) living in polluted environments. Ital. J. Zool. 2005, 72, 285–292. [Google Scholar] [CrossRef]
- Hughes, G. Introduction to the study of gills. Semin. Ser.-Soc. Exp. Biol 1982, 16, 1–24.ill. [Google Scholar]
- Evans, D.H. The fish gill: Site of action and model for toxic effects of environmental pollutants. Environ. Health Perspect. 1987, 71, 47–58. [Google Scholar] [CrossRef]
- Srivastava, N.; Kumari, U.; Rai, A.K.; Mittal, S.; Mittal, A.K. Alterations in the gill filaments and secondary lamellae of Cirrhinus mrigala exposed to “nuvan,” an organophosphorus insecticide. J. Histol. 2014, 190139. [Google Scholar] [CrossRef] [Green Version]
- Seok, S.-H.; Baek, M.-W.; Lee, H.-Y.; Kim, D.-J.; Na, Y.-R.; Noh, K.-J.; Park, S.-H.; Lee, H.-K.; Lee, B.-H.; Ryu, D.-Y.; et al. Quantitative GFP fluorescence as an indicator of arsenite developmental toxicity in mosaic heat shock protein 70 transgenic zebrafish. Toxicol. Appl. Pharmacol. 2007, 225, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Rajini, A.; Revathy, K.; Selvam, G. Histopathological changes in tissues of Danio rerio exposed to sub lethal concentration of combination pesticide. Indian J. Sci. Technol. 2015, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Vutukuru, S.S. Assessment of the Histological Changes in the Gill Architecture of Zebrafish (Danio rerio) Exposed to Nicotine and Cotinine Extracts from Cigarette Tar. EC Pharmacol. Toxicol. 2019, 7, 888–895. [Google Scholar]
- Ertzer, R.; Müller, F.; Hadzhiev, Y.; Rathnam, S.; Fischer, N.; Rastegar, S.; Strähle, U. Cooperation of sonic hedgehog enhancers in midline expression. Dev. Biol. 2007, 301, 578–589. [Google Scholar] [CrossRef]
- Park, H.; Lee, J.-Y.; Park, S.; Song, G.; Lim, W. Developmental toxicity and angiogenic defects of etoxazole exposed zebrafish (Danio rerio) larvae. Aquat. Toxicol. 2019, 217, 105324. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, J.; Han, X.; Huang, T. The use of zebrafish (Danio rerio) behavioral responses in identifying sublethal exposures to deltamethrin. Intl. J. Environ. Res. Public Health 2014, 11, 3650–3660. [Google Scholar] [CrossRef] [Green Version]
- Dabrowski, K.; Miller, M. Contested Paradigm in Raising Zebrafish (Danio rerio). Zebrafish 2018, 15, 295–309. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.B.; Schwab, T.L.; Sigafoos, A.N.; Gauerke, J.L.; Krug, R.G., 2nd; Serres, M.R.; Jacobs, D.C.; Cotter, R.P.; Das, B.; Peterson, M.O.; et al. Novel zebrafish behavioral assay to identify modifiers of the rapid, nongenomic stress response. Genes Brain Behav. 2019, 18, e12549. [Google Scholar] [CrossRef] [PubMed]
- De Marco, R.J.; Groneberg, A.H.; Yeh, C.-M.; Treviñ, M.; Ryu, S. The behavior of larval zebrafish reveals stressor-mediated anorexia during early vertebrate development. Front. Behav. Neurosci. 2014, 8, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, K.R.; Johnson, I.T.; Fenwick, G.R. The chemistry and biological significance of saponins in foods and feedingstuffs. Crit. Rev. Food Sci. Nutr. 1987, 26, 27–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Di iorgio, C.; Lamidi, M.; Elias, R.; Ollivier, E.; De Meo, M.P. Genotoxic and clastogenic activity of saponins extracted from Nauclea bark as assessed by the micronucleus and the comet assays in Chinese hamster ovary cells. J. Ethnopharmacol. 2011, 137, 176–183. [Google Scholar] [CrossRef]
- Yim, S.-H.; Tabassum, N.; Kim, W.H.; Cho, H.; Lee, J.H.; Batkhuu, G.J.; Kim, H.J.; Oh, W.K.; Jung, D.W.; Williams, D.R. Isolation and characterization of isofraxidin 7-O-(6′-Op-Coumaroyl)-β-glucopyranoside from Artemisia capillaris Thunberg: A novel, nontoxic hyperpigmentation agent that is effective in vivo. Evid. Based Complement. Altern. Med. 2017, 1401279. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.H.; Yang, Z.S.; Wen, C.C.; Chang, Y.S.; Wang, B.C.; Hsiao, C.A.; Shih, T.L. Evaluation of the structure-activity relationship of flavonoids as antioxidants and toxicants of zebrafish larvae. Food Chem. 2012, 134, 717–724. [Google Scholar] [CrossRef]
- Yoon, Y.; Park, J.; Taniguchi, A.; Kohsaka, H.; Nakae, K.; Nonaka, S.; Ishii, S.; Nose, A. System level analysis of motor-related neural activities in larval Drosophila. J. Neurogenet. 2019, 33, 179–189. [Google Scholar] [CrossRef]
- Chen, Y.H.; Huang, Y.H.; Wen, C.C.; Wang, Y.H.; Chen, W.L.; Chen, L.C.; Tsay, H.J. Movement disorder and neuromuscular change in zebrafish embryos after exposure to caffeine. Neurotoxicol. Teratol. 2008, 30, 440–447. [Google Scholar] [CrossRef]
- Mendis, J.C.; Tennakoon, T.K.; Jayasinghe, C.D. Zebrafish embryo toxicity of a binary mixture of pyrethroid insecticides: D-tetramethrin and cyphenothrin. J. Toxicol. 2018, 2018, 4182694. [Google Scholar] [CrossRef]

| Cells | Fruit Fly | Zebrafish | Rodents | Humans | |
|---|---|---|---|---|---|
| Benefits | Fast, simple, inexpensive | Short period of generation | Fast, simple, inexpensive | Complexity of body | High value translation |
| Well-standardized | Work easily with | Complexity of body | Adequate predictiveness | Credible and logical | |
| Many probable readings | Low maintenance costs | Ethical examination in vivo | Administration route | Dosage details | |
| Limitations | Modest predictivity | Genetically far from human beings | Modest flexibility | Time-consuming and costly | Time-consuming and costly |
| Simplified process | Simple anatomy | Moderate foresight | Comprehensive regulation | Comprehensive regulation | |
| Low value translation | No efficient immune system | Moderate value translation | Ethical limits | Ethical limits |
| Life Stage | Measured Endpoints | References |
|---|---|---|
| Egg | Survival, hatching, teratology | [1,60,67] |
| Larvae | Survival, abnormalities, enzyme activities and behavior | [67,68,69,70] |
| Adults | Survival, behavior, enzyme activities and genotoxicity | [71,72] |
| Plant/Family | Common Name | Extraction/Part of Plant | Concentrations Range (µg/mL) | LC50 (µg/mL) | Characteristics of Effect | Reference |
|---|---|---|---|---|---|---|
| Acorus calamus/Acoraceae | Sweet flag | Ethanol/rhizome | n.a. | n.a. | Fish treated with this extract returned to normal condition and revealed a decrease level in superoxide dismutase and decreased mitochondrial volume and cell viability. | [74] |
| Achyranthes aspera/Amaranthaceae | Chaff-flower, prickly chaff flower, devil’s horsewhip, burweed | Ethanol/leaf | n.a. | n.a. | Fish treated with this extract returned to normal condition and revealed decrease in superoxide dismutase level and decreased mitochondrial and cell viability. | [74] |
| Achyranthes bidentate/Amaranthaceae | Ox knee, niu xi (Chinese) | Aqueous/root | 0–30.01 µg/mL | No mortality observed | The extract did not exhibit noticeable lethal or severe side effects on zebrafish larvae/embryos. | [75] |
| Allium carinatum/Amaryllidaceae | Keeled garlic, witch’s garlic | 70% aqueous methanol/whole plant | 1–60 µg/mL | 55.8 µg/mL | The extract reduced developmental toxicity and neutropenia. No teratogenic effects were seen. | [76] |
| Allium flavum/Amaryllidaceae | The small yellow onion, yellow-flowered garlic | 70% aqueous methanol/whole plant | 1–60 µg/mL | 50.3 µg/mL | The extract reduced developmental toxicity and neutropenia. No teratogenic effects were seen. | [76] |
| Andrographis paniculata/Acanthaceae | Green chiretta, creat | Aqueous/leaf | 0–10,000 µg/mL | 525.5 µg/mL (48 hpf) 525.6 µg/mL (96 hpf) | Morphological defects were observed at 96 hpf after exposure to teratogen concentration. | [2] |
| Annona squamosa/Annonaceae | Sugar apple, sweetsop | Ethanol/young leaf | 100–1000 µg/mL | n.a. | Late hatching process and morphological deformations occurred at higher concentrations up to 800 µg. | [77] |
| Avicennia marina/Acanthaceae | Grey mangrove or white mangrove | Methanol/bark, leaf, stem, flower and fruit | 50–100 µg/mL | n.a. | n.a. | [78] |
| Azadirachta indica/Meliaceae | Neem | Ethanol (cold)/fruit | 1000–5000 µg/mL | n.a. | Not toxic to adult zebrafish and did not alter the locomotor system. | [79] |
| Bougainvillea galbra/Nyctaginaceae | The lesser bougainvillea, paperflower | Aqueous/bracts | 1–300 µg/mL | 85.51 µg/mL | Yolk sac edema was observed at different concentrations. Hypopigmentation in the embryo was caused by the purple bract extract at 30 µg/mL. Overall, this extract generally showed moderate embryo toxicity. | [80] |
| Bixa Orellana/Bixaceae | Achiote | Hot water/leaf | 50–10,000 µg/mL | n.a. | Plant extract affected the hatchability of embryos and was linked to delayed development. Also, a coagulated embryo, and tail malformation were observed as teratogenic effects. | [81] |
| Capsicum chinense/Solanaceae | Habanero type pepper | Ethanol/fruit | 0.39–100 µg/mL | 39.7 ± 2.1 µg/mL | The embryo demonstrated late development represented by an absence of pigmentation of the tail, and irregular formation of the somites demonstrated the noticeable tail curve with a wide end. | [82] |
| Carthamus tinctorius/Asteraceae | Safflower (English), Kashefeh (Persian) | Aqueous/flower | 40–1000 µg/mL | 345,600 µg/L | Hatching inhibition, depressed heart rate, abnormal spontaneous movement, pericardial edema, yolk sac edema, unusual head-trunk tilt, suppression of melanin release, enlarged yolk, and short body length were identified. | [83] |
| Clinacanthus nutans/Acanthaceae | Sabah snake grass | Hexane/leaf | 15.63–500 µg/mL | 75.49 µg/mL | Morphological disorders (less pigmentation, tail bending, edema, abnormal yolk sac). | [84] |
| Cinnamon zeylanicum/Lauraceae | True cinnamon tree, Ceylon cinnamon | Aqueous/bark | 0–10,000 µg/mL | 985.8 µg/mL (48 hpf) 50.58 µg/mL (96 hpf) | This plant showed minimum embryotoxicity on zebrafish. In 72 hpf embryos, higher concentration of extract provoked yolk sac and pericardial edema. | [2] |
| Croton tiglium/Euphorbiaceae | Purging croton, Jamaal gota (Hindi) | Aqueous/seed | 4000–24,000 µg/mL | 11,880 µg/mL (24 h) 8160 µg/mL (48 h) | Signs of stress, enhanced respiratory rate, jerky movements, loss of equilibrium, circular swimming before losing balance. | [10] |
| Curcuma longa/Zingiberaceae | Turmeric | Methanol/rhizome | 7.8–125 µg/mL | 92.42 µg/mL (24 hpf) 79.20 µg/mL (48 hpf) 68.32 µg/mL (72 hpf) 56.68 µg/mL (96 hpf) 55.90 µg/mL (120 hpf) | Physical anatomy deformities as teratogenic effects; tail bending, yolk sac edema extension, and a curved trunk were observed at high concentration among hatched embryos. | [1] |
| Curcuma longa/Zingiberaceae | Turmeric | Methanol/rhizome | No concentration ranges were mentioned (10 µg/mL used) | n.a. | No visible symptoms of toxicity were observed: no change in heart rate, body impairment, absence or delay in reaction to tactile stimuli, or mortality. | [85] |
| Curcuma xanthorrhiza/Zingiberaceae | Javanese ginger, Temulawak | Ethanol/rhizome | 100–500 µg/mL | 180.52 µg/mL at 48 h 79.55 µg/mL at 96 h | Major malformations of the pericardial edema of embryos at a concentration of 100 µg/mL, yolk sac edema and tail malformation also were observed. | [43] |
| Javanese ginger, Temulawak | Aqueous/rhizome | 0–10,000 µg/mL | 748.6 µg/mL (48 hpf) 703.7 µg/mL (96 hpf) | Decreased survival rate, organ deformity, unusual heartbeat, and delayed hatching rates. | [2] | |
| Cynodon dactylon/Poaceae | Scutch grass | Hexane, chloroform, acetone, methanol/n.a. | 10–100 µg/mL | 32.6 µg/mL | The period of systole and diastole was decreased by the methanol extract. | [86] |
| Derris elliptica/Fabaceae | Tuba root (Indonesia), opay (Philippines) | Aqueous/leaf | 50 (0.05%), 500 (0.5%) µL/mL | n.a. | The concentration of 0.5% led to the early death of embryos: unformed head, unformed tail, coagulation, and death. A delay in growth and restricted movement compared to the control group were present at 0.05%. | [53] |
| Dieffenbachia amoena/Araceae | Spotted dumbcane | Aqueous/leaf | 0–10,000 µg/mL | 1190 µg/mL | Growth retardation, no heartbeat was noticed at 500 µg/mL and higher concentrations (cardiotoxicity). | [87] |
| Diospyros discolor/Ebanaceae | Velvet persimmon, velvet apple, mabolo tree | Aqueous/leaf | 0.05–10% | 1% | Tail malformation, delayed growth, head abnormality, yolk malformations, and abdominal swelling were observed. | [88] |
| Eclipta prostrata/Asteraceae | False daisy, yerba de tago, Karisalankanni and bhringraj | Aqueous/leaf | 0.01–40% | 1% | At the concentration of 0.01% v/v and 1% v/v, did not observe any mortality and abnormality | [89] |
| Enydra fluctuans/Asteraceae | Marsh herb, water cress | Ethanol/leaf | 12,500–400,000 µg/mL | 24 h: 204,132 µg/L 48 h: 170,513 µg/L 72 h: 139,478 µg/L 96 h: 92,956 µg/L | The death rate was found as the exposure period was extended from 24 to 96 h, the median lethal concentration decreased. A negative relationship between exposure time and LC50 was found. | [90] |
| Eugenia polyantha/Myrthaceae | Bay leaf | Aqueous/leaf | 0–10,000 µg/mL | 921.2 µg/mL (48 hpf) 60.39 µg/mL (96 hpf) | Decreased survival rate, organ deformity, unusual heartbeat, and delayed hatching rates. | [2] |
| Euphorbia pekinensis Rupr/Euphorbiaceae | The Peking spurge, daji (Chinese) | Aqueous/commercial raw herb | 100–250 µg/mL | 250–300 µg/mL (previous study) | n.a. | [91] |
| Euodia rutaecarpa (Tetradium ruticarpum)/Rutaceae | Goshuyu (Japanese), Bee tree | Evodiamine (Commercial product) | 0.05–1.6 µg/mL | 0.354 µg/mL (LC10) | Evodiamine (bioactive compound) had a 10% lethality at 354 ng/mL and caused heart defect, changes in heartbeat and blood flow, and pericardial malformation. Also, it could cause cardiovascular side effects involving oxidative stress. | [92] |
| Excoecaria agallocha/Euphorbiaceae | Back mangrove | Methanol/bark, leaf and stem | 50–100 µg/mL | n.a. | Higher concentrations induced intense impacts on embryo melanogenesis. Those concentrations also reduced the eye melanin material of the embryo. | [78] |
| Ficus glomerate/Moraceae | Cluster fig tree, Indian fig tree, goolar fig | Hot aqueous/leaf | 125–2000 µg/mL | 239.88 µg/mL | Lower hatchability, body size, heartbeat rate, and morphological growth defects of the embryo were exhibited. | [73] |
| Garcinia hanburyi/Clusiaceae | Siam gamboge, Hanbury’s Garcinia | Commercial product/dried yellow resin | 0.5–1.0 µM | 1.76 µM | Gambogic acid triggered a lack of fin developmental in the zebrafish embryo. | [93] |
| Garcinia mangostana (Xanthone crude extract)/Clusiaceae | Mangosteen | Mixture of acetone and water (80:20) | 7.81–250 µg/mL | 15.63 µg/mL | Among surviving zebrafish, no deformities were found. | [28] |
| Geissospermum reticulatum/Apocynaceae | Challua caspi (Kichwa language) | Ethanol/bark | 0.1 µg/mL | n.a. | This plant was nontoxic and caused no deformations to zebrafish, even at high concentrations. | [94] |
| Himatanthus drasticus/Apocynaceae | Janaguba milk (latex) | Commercial product | 500–1500 µg/mL | 1188.54 µg/mL | This plant can be considered to have low toxicity. No teratogenic effect was observed. | [95] |
| Hylocereus polyrhizus/Cactaceae | Pitaya | Ethanol; water solution (70:30, v/v)/peel & pulp | 100–1000 µg/mL and 20 µL) | >1000 µg/mL (96 h) | The results indicated the pulp and peel of this plant were non-toxic. | [96] |
| Leonurus japonicus/Lamiaceae | Oriental/Chinese motherwort | Essential oil/the aerial parts | 6.25–100 µg/mL | 1.67 ± 0.23 µg/mL | Motherwort essential oil was toxic to embryos. Morphological abnormalities: partial or complete absence of eye development, yolk sac oedema, curved spine, tail deformities, scattered haemorrhages in the oedematous yolk sac, incomplete heart development, and pericardial oedema. | [97] |
| Ligusticum chuanxiong/Apiaceae | Szechuan lovage | A protein-containing polysaccharide/rhizome | 0–800,000 µg/L | 965 µg/mL | No significant morphological anomalies were found. | [98] |
| Maerua subcordata (Gilg) DeWolf/Capparaceae | Methanol/fruit, leaf, root tuber, and seed | 150,000–1,500,000 µg/L | 209,000 µg/L | ≤5% sub-lethal abnormalities (signs of malformation of the heart). | [4] | |
| Marsdenia tenacissima (Xiaoaiping extract)/Asclepiadaceae | Murva (Hindi), Tong-Guan-Teng (Chinese) | Commercial product | 0–3200 µg/mL | 2660 µg/mL (24 hpf) 2310 µg/mL (48 hpf) 1920 µg/mL (72 hpf) 1910 µg/mL (96 hpf) 1790 µg/mL (120 hpf) | Severe malformation such as impairment of the swim bladder, retaining the yolk, pericardial oedema, and tail curvature. Histopathological study: Xiaoaiping induced lesion of the liver, muscle, and heart. | [99] |
| Millettia pachycarpa/Fabaceae | Bokol-bih, Holsoi, Bokoa-bih (Assamese or Hindi) | Aqueous/root | 1–7.5 µg/mL | 4.276 µg/mL | There were a few developmental anomalies, such as yolk sac oedema, curvature of the spinal cord, pericardial oedema, swim bladder swelling, reduced heart rate, and late hatching. This plant extract is also linked to reactive oxygen species and apoptosis, which induced embryonic mortality and toxicity (identified in trunk, brain, and tail). | [6] |
| Momordica charantia/Cucurbitaceae | Bitter melon, bitter gourd, bitter apple | Methanol/fruit, seed | 1–400 µg/mL | 50 µg/mL (only seed extract) | Although it affected the heartbeat, the fruit extract is considered to be harmless and no mortality was observed. The seed extract demonstrated a slight level of developmental failure and had significant cardiac toxicity. | [100] |
| Hot and cold aqueous/leaf | 15.625–1000 µg/mL | 144.54 µg/mL: Hot aqueous Chinese extract 199.53 µg/mL: Hot aqueous Indian extract 251.19 µg/mL: Cold aqueous Chinese extract | For all samples the heart beat differed at higher concentrations. Also, mild toxicity was observed, although consumption of this plant is assumed to be safe. | [12] | ||
| Aqueous/leaf | 0.05–3% | 3.0% at 24 h | Teratogenic effects such as bent back, tip of tail bent, oedema in the yolk sac, and scoliosis. Delayed development and morphological deformities of the embryo were observed. | [101] | ||
| Moringa oleifera/Moringaceae | Drumstick tree, Moringa | Hot water/leaf and bark | 0.3–6 µg/mL | 1.5 µg/mL at 36 h (leaves); 3 µg/mL at 36 h (bark) | Even low concentration was embryo-toxic and had teratogenic impacts on the developing embryos. | [102] |
| Essential oil/seed | 0.1–1000 µg/mL | 21.24 ± 0.44 µg/mL | High concentrations (≥ 50 µg/mL) caused 100% mortality of embryos. The process of angiogenic blood vessel formation in zebrafish embryos was substantially disturbed by the seed oil. | [103] | ||
| Aqueous/seed | 12.5–200 µg/mL | 190 µg/mL (48 h) 133 µg/mL (72 h) 49 µg/mL (96 h) | No toxic endpoint was seen after 24 h, hatching was prolonged, and the larval period at 96 h compared with the control was decreased. | [104] | ||
| Moringa peregrina/Moringaceae | Miracle tree | Essential oil/seed | 0.1–1000 µg/mL | 25.11 ± 0.547 µg/mL | High concentrations (≥ 50 µg/mL) caused 100% mortality of embryos. The process of angiogenic blood vessel formation in zebrafish embryos was substantially disturbed by the seed oil. | [103] |
| Olea europaea/Oleaceae | Olive | Raw and polar fraction (raw oil: commercial)/fruit | 1–100% | Raw oil: 11.98% (EC50) Polar fraction: 61.87 (EC50) | Abnormalities observed included non-hatching, pericardial oedema, and blood accumulation, and more were expressed at a concentration of 11.98%. Raw oil induced 50% of developmental abnormalities compared with the polar fraction. After 96 h, strong developmental retardations such as developmental delay and absence/prolongation of pigmentation formation were observed. | [105] |
| Onosma dichroantha/Boraginaceae | n.a. | Cyclohexane, ethyl acetate, methanol/root | 0.02–50 µg/mL | n.a. | n.a. | [106] |
| Orthosiphon stamineus/Lamiaceae | Java tea | Aqueous/whole plant | 0–10,000 µg/mL | 1685 µg/mL (48 hpf) 1685 µg/mL (96 hpf) | n.a. | [2] |
| Palicourea deflexa/Rubiaceae | n.a. | Methanol (fraction)/leaf | 1–100 µg/mL | 72.18 µg/mL | At high concentration (100 µg/mL), some possible anomalies, such as no development of somites, decreased pigmentation, and formation of oedema were observed. Lethality only appeared after 96 h. | [52] |
| Palmaria palmata/Archaeplastida | Dulse, dillisk (dilsk), red dulse, sea lettuce flakes (creathnach) | Crude protein (hydrolysate)/leaf | 1–10,000 µg/mL | n.a. | At high concentration (10 mg/mL), larvae started developing spinal curvature and a deformed yolk sac. At 5 mg/mL, larvae appeared to have a swollen yolk sac and developed a curved spine. | [51] |
| Passiflora caerulea/Passifloraceae | Bluecrown Passionflower | Aqueous/leaf | 40–120 µg/mL | 80 µg/mL (40–120 µg/mL) | Mortality increased based on concentration and delay in hatching. No apparent signs of abnormal growth or morphology were detected in embryos at 96 hpf. | [107] |
| Peucedanum alsaticum/Apiaceae | n.a. | n-heptane, ethyl acetate, methanol, water/fruit | 50–400 µg/mL | n.a. | n.a. | [33] |
| Phyllanthus niruri/Phyllanthaceae | Gale of the wind, stonebreaker | Aqueous/leaf | 0.05–10% | – | Tail malformation and coagulation were detected as the most remarkable toxic effect from the extract; dose-dependent impacts on the heartbeat and hatchability of the embryo also were detected. | [108] |
| Piper betle/Piperaceae | Betel, Ikmo, Daun sirih (Malay), Paan (Hindi) | Hot water/leaf | 50–10,000 µg/mL | – | The plant extract affected the hatchability of embryos and was linked to delayed development. Also, a coagulated embryo and tail malformation were observed as a teratogenic effect. | [81] |
| Polygonum multiflorum/Polygonaceae | Tuber fleeceflower | n-hexane, ethyl acetate, water/root | 0–175,000 µg/L | TIs: 1430 (ethyl acetate), 630 µg/L | 40 and 105 mg/L concentrations (all extracts) induced yolk sac oedema (heart oedema), hemovascular defects, abnormal trunk, and necrosis. The water extract induced synthesis of melanin in zebrafish through stimulation of tyrosinase. | [109] |
| Water, ethanol, methanol, acetone/dried roots | 0–10,000 µg/mL | 129.4 ± 2.7 µg/mL (water) 58.2 ± 1.9 µg/mL (30% ethanol) 39.8 ± 0.8 µg/mL (50% ethanol) 27.9 ± 2.3 µg/mL (70% ethanol) 25.5 ± 2.0 µg/mL (95% ethanol) 48.8 ± 2.8 µg/mL (methanol) 23.6 ± 2.2 µg/mL (acetone) | Teratogenic effects included coagulated embryos, lack of development of the somite, absence of tail detachment and heartbeat, and malformation of the notochord | [110] | ||
| Psoralea corylifolia/Fabaceae | Babchi | Commercial product (Psoralen) | 0–6.70 µg/mL | 3.40 µg/mL (LC50) 2.52 µg/mL (LC10) 1.98 µg/mL (LC1) | Psoralen therapy caused hatching rate and body size to decrease and the abnormality rate of zebrafish to increase significantly. Yolk retention, pericardial oedema, swim-bladder malformation, and curved body form were observed. | [111] |
| Punica granatum/Lythraceae | pomegranate | Ethanol/peel | 100–250 µg/mL | 196,037 ± 9.2 µg/mL | No issues with the reproductive organs, heart, and androgen hormones were detected. | [112] |
| Rhizosphora apiculata/Rhizosphoraceae | Mangrove | Methanol/bark, leaf, stem and root | 50–100 µg/mL | n.a. | Higher concentrations of the extract from bark, leaf, and stem did not cause mortality. | [78] |
| Salvia miltiorrhiza/Lamiaceae | Red sage | Commercial product/Tanshinone IIA (diterpene quinone)/root | 0.44–7.06 µg/mL | 5.44 µg/mL (72 hpf) 3.77 µg/mL (96 hpf) | Tan-IIA showed potential cardiotoxicity and growth inhibition in zebrafish embryos. Scoliosis, tail deformity, and pericardium oedema were the primary signs of teratogenicity. | [45] |
| Sida acuta/Malvaceae | Wireweed | Hexane, chloroform, acetone, methanol/n.a. | 10–100 µg/mL | 20.9 µg/mL | The methanolic extract led to heartbeat rate reductions (more significant than nebivolol as a positive control). This study reported no teratogenic effects. | [86] |
| Solidago canadensis/Asteraceae | Canada goldenrod, Canadian goldenrod | Ethanol/leaf | 0–500µg/mL | 0.42 ± 0.03 µg/mL (24 h) 0.33 ± 0.04 µg/mL (48 h) 0.32 ± 0.03 µg/mL (72 h) | This paper only mentioned that low toxicity on zebrafish was observed. | [113] |
| Sonneratia alba/Lythraceae | Mangrove tree | Methanol/bark and leaf | 50–100 µg/mL | – | – | [78] |
| Spilanthes acmella/Asteraceae | Paracress, toothache plant, Sichuan buttons, buzz buttons, tingflowers, electric daisy Kradhuawean (Thai) | Aqueous/leaf | 0.01–20% | n.a. | No mortality was observed in the highest concentration test. | [89] |
| Spondias mombin/Anacardiaceae | Yellow mombin, hog plum | Hydroethanolic/leaf | 1–9 g/kg (LD50); 25,000–75,000 µg/L (LC50) | 4.515 g/kg (LD50: 48 h: immersion) 49.86 µg/mL (LC50: 48 h: oral) | There were no recorded teratogenic effects. | [114] |
| Streblus asper/Moraceae | Siamese rough bush, khoi, serut, thoothbrush tree | Methanol/bark | 50–100 µg/mL | 2,000,000 µg/mL | Slight oedema of heart muscles at 100 µg/mL. No other severe malformations were observed. | [115] |
| Sutherlandia frutescens/Fabaceae | Cancer bush, balloon pea, sutherlandia | Aqueous and 80% ethanol | 5–50 µg/mL | 30.00 µg/mL | Both extracts showed bleeding and pericardial cyst development at high concentration, but the aqueous extract was less toxic to larvae. | [8] |
| Aqueous, ethanol/aerial part | 5–300 µg/mL | 297.57 µg/mL (aqueous) 40.54 µg/mL (ethanol) | The high concentration of both extracts inhibited hatching rate and mortality. | [116] | ||
| Terminalia chebula/Combretaceae | Myrobalan | Ethanol/fruit, leaf, root | n.a. | n.a. | At 50 µg/L, fish treated with this extract came back to normal condition and showed a related decrease in superoxide dismutase and decreased mitochondria and cell viability. | [74] |
| Tetrapterys (Melanolepis) multiglandulosa/Malpighiaceae | Pakalkal (Tagalog) | Dichloromethane, hexane, ethyl acetate, methanol and water/leaf | n.a. | 200 µg/mL (methanol extract) | A significant ecdysteroid was the most toxic in the zebrafish. | [117] |
| Tinospora cordifolia/Menispermaceae | Heart-leaved moonseed, gaduchi, guduchi, giloy, Makabuhay (Filipino) | Aqueous/leaf and bark | 0.01–10% | 1% (leaves), 10% (barks) | Abnormalities of head and tail, delayed development, reduced mobility, stunted tail, and scoliosis/flexure were observed. | [118] |
| Thuja orientalis/Cupressacae | Oriental Arbor-vitae | Ethanol/leaf | 150–2400 µg/mL | 702.9 µg/mL | At 2.4 mg/mL embryos were coagulated; at 1.2 mg/mL they did not hatch and oedema was observed; 0.6 mg/mL resulted in skeletal deformities; at 0.3 mg/mL slight oedema was observed; at 0.15 mg/mL normal growth was observed. | [50] |
| Trapa natans/Lythraceae | Water chestnut, water caltrop | Acetone, methanol and ethyl acetate/leaf | 100,000–1,000,000 µg/mL | 50 µg/mL (methanol) 40 µg/mL (acetone) 30 µg/mL (ethyl acetate) | Methanol and acetone extracts did not show toxicity to zebrafish. | [119] |
| Tripterygium wilfordii/Celasteraceae | Thunder god vine, léi gōng téng (Mandarin) | Purchased commercially each compound | 1/10, 1/5, 1/2 LC50 | Taxol: 0.10, 0.21, 0.53 µg/mL; Gambogic acid 0.03, 0.05, 0.13 µg/mL; Triptolide: 0.04, 0.07, 0.18; Auranofin: 0.84, 1.68, 4.21 µg/mL; Mycophenolic acid: 0.44, 0.95, 2.22; Curcumin: 0.43, 0.87, 2.17 µg/mL; Thalidomide: 0.71, 1.43, 3.56 µg/mL | From those 7 compounds, gambogic acid followed by taxol showed high anticancer activity | [120] |
| Zanthoxylum sp./Rutaceae | n.a. | Commercial product; zanthoxylum | 0–1000 µg/mL | 81.18 µg/mL | No teratogenic effects were reported. | [121] |
| Exposure Format | Number | Exposure Stage | Exposure Time | Parameters Evaluated (Endpoint) | Compound(s) | Reference |
|---|---|---|---|---|---|---|
| 96-well plates | 1/well | 24 hpf | 96 h | Embryotoxicity | Armatamide, rubecenamide, lemairamin, rubemamine, and zanthosine | [121] |
| 1/well (10/group) | 6 hpf | 96 h | Embryotoxicity and teratogenic effects | Palmitic acid, phytol, hexadecanoic acid, 1-monopalmitin, stigmast-5-ene, pentadecanoic acid, heptadecanoic acid, 1-linolenoylglycerol, and stigmasterol | [84] | |
| 30/group (90 embryos) | n.a. | 96 h | Teratogenic effects and embryotoxicity | Alkaloids, flavonoids, phenolics, and saponins | [73] | |
| 1/well | 24 hpf | 72 h | Acute toxicity assay | Polysaccharides, peptides, protein, lipids, terpenoids, saponins, phenolics, and sterols | [12] | |
| 12/group | 2 hpf | 96 h | Embryotoxicity and teratogenic effects | α/β-thujone, thujone | [50] | |
| n.a. | 1 hpf (embryo) | 72 h | Cytotoxicity and embryonic toxicity test (survival rate) | Xanthone | [28] | |
| 4/group (per well) | 12 hpf | 48 h | Embryotoxicity (mortality, hatchability, heartbeat rate, and malformation), teratogenic activity | Ascorbic acid, flavonoids, phenolics, and carotenoids | [102] | |
| 1/well | 24 hpf | 96 h | Embryotoxicity and teratogenic effects | Capsaicin, ethyl palmitate, ethyl linoleate, dihydrocapsaicin, and docosenamide | [84] | |
| 36/group | 2.5–3.7 hpf | 72 h | Developmental toxicity and behavioural safety | Roots: glycoside, siaraside (pregnane glycoside); Stem bark: α-amyrin, acetate, lupeol, and β-sitosterol | [115] | |
| 12/group | 4 cell embryo stage | 144 h | Embryotoxicity (heart rate and hatching time) | Spilanthal, and flavonoid | [89] | |
| 24-well plates | 12 fertilized eggs/group | 6 hpf | 120 h | Embryotoxicity and teratogenic effects (hatching rate, heartbeat, otolith, blood circulation, tail detachment, and motility) | Catechin, epicatechin, and naringenin | [1] |
| 20/concentration | 6 hpf | 96 h | Embryotoxicity | [76] | ||
| 10/well | 6 hpf | 96 h | Embryotoxicity | Methanol extract: phenolic compound (p-heydroxybenzoic acid), phenolic acid, flavonoid, and flavonoid glycoside | [119] | |
| 10/group (30 per tested sample) | 64-cell (egg) | 96 h | Embryotoxicity | Phenolic compounds: tyrosol, catechol. Hydroxytyrosol, gallic acid, and ascorbic acid. | [105] | |
| 1/well | 4 hpf | 96 h | Embryotoxicity | Pyrrolizidine alkaloids such as senecionine or senecivernine; phenolic compounds, terpenoids, and iridoids, glucosinolates (glucolepidin and glucobrassicin), alkaloids or amines (stachydrine and trigonelline) | [4] | |
| 4/group | 12 hpf | 48 h | Embryotoxicity, teratogenic effects | n.a. | [108] | |
| 30/group | 4 hpf | 96 h | Embryotoxicity | Phenols, and polyphenols | [107] | |
| 10/well | 16 cell stage | 72 h | Embryotoxicity | n.a. | [82] | |
| 6/well | 24 hpf | 96 h | Embryotoxicity | Sutherlandin, and sutherlandioside | [116] | |
| 6/group (per well) (n =18) | 24 hpf | 216 h | Developmental toxicity and cardiotoxicity | No pure compound isolated | [8] | |
| 15/well | 2 hpf | 96 h | Embryotoxicity, teratogenic effects | Alkaloids (stachydrine) | [97] | |
| 4/well | – | 48 h | Embryotoxicity, teratogenic effects | n.a. | [88] | |
| n.a. | 6 hpf | 96 h | Acute toxicity in vivo (zebrafish), in silico (computational chemistry) | Brevifolin, tannin, saponin, quinone, steroid/triterpenoid, and flavonoid | [112] | |
| 15/well | 4 hpf | 96 h | Embryotoxicity, teratogenic effects | Psoralen | [111] | |
| 40/group | 16-cell stage | 96 h | Zebrafish embryo acute toxicity (ZFET) | Emodin, rhein, physcion (dihydroxyanthraquinone), chrysophanol, aloe-emodin, rhaponticin, polygonumnolide, and 2,3,5,4’-tetrahydroxystilbene-2-O-β-D-glucopyranosede, and resveratrol-2-O-β-D-glucopyranoside | [110] | |
| 30/group (10/well) | 9 hpf | 55 h | Anti-melanogenic and embryotoxicity | Lupeol (triterpenoid), hydrolysable, and 3,3′-di-O-methyl ellagic acid (bark of S. alba) | [78] | |
| 1/well (20 embryos per concentration) | 16-cell stage | 168 h | Embryotoxicity | n.a. | [104] | |
| 20 eggs/group (1 embryo per well | 1 hpf (embryo) | 96 h | Embryotoxicity, teratogenic effects (body axis, head, tail, blood circulation, eyes, heart, pigmentation, somite, and yolk sac) | Curcumin, desmethoxycurcumin, and bisdemethoxycurcumin | [43] | |
| 1/well | 32-cell stage | 96 h | Embryotoxicity | harman-3-carboxylic acid, βc alkaloids: β-carboline alkaloid | [52] | |
| 30 embryos/well | 2 hpf | 96 h | Toxicity on cell and zebrafish (coagulation of eggs, tail detachment, presence of heartbeat, and hatching rate) | Alkanes, alkenes, amino acid, amines, and amides | [2] | |
| 20/well | 6 hpf | 72 h | – | 3-O-propionyl-5, 10, 14-O-triacetyl-8-O-(20 -methyl- butanoyl)-cyclomyrsinol, and mirsinance type diterpene | [91] | |
| 20/well | 2 hpf | 70 | Embryotoxicity, teratogenic effects | Gambogic acid | [93] | |
| 5/group (per well) | 6 hpf | 120 h | Developmental toxicity and apoptosis induction | Rotenone, and saponin | [6] | |
| 10/well | 24 hpf | 96 h | Embryotoxicity | n.a. | [86] | |
| 6/well | 7 dpf | 24 h | Determination of maximum tolerated concentration (MTC) | ar-turmerone, α, β-turmerone, and α-atlantone | [85] | |
| 12-well plates | 4/group (per vial) | 1 hpf (embryo) | 48 h | Embryotoxicity, teratogenic effects | Beta-momorcharin | [101] |
| 20/group | 6 hpf | 96 h | Embryotoxicity, teratogenic effects | Quinochalcones, and flavonoids | [83] | |
| 4/well | – | 48 h | Embryotoxicity, teratogenic effects | Flavonoids, terpenoids, cardiac glycosides, saponins, and tannins | [81] | |
| 4/group (per well) | 12 hpf | 48 h | Embryotoxicity, teratogenic effects | Alkaloids, di-terpenoid lactones, glycosides, steroids, sesquiterpenoid, phenolics, and aliphatic | [118] | |
| 3/well | n.a. | 48 h | Embryotoxicity and teratogenicity | Rotenone | [53] | |
| 10/well | 48 hpf | 48 h | Embryotoxicity | Peptides | [51] | |
| 12/group | egg | 120 h | Developmental toxicity (mortality, hatching rate, oedema, and malformations) | Alkaloids, flavonoids, terpene derivatives, glycosides, novel diazepine, and squalene | [77] | |
| Glass beaker | 24/group | n.a. | 96 h | Acute toxicity test | Phyllocactins, betanins, 2′-O-apiosyl-malonyl-betanin, 6′-O-malonyl-2-descarboxybetanin, flavonoids, isorhamnetin triglycosides, quercetin-3-O-hexoside, and carbohydrates. | [96] |
| 15/group | 0 hpf | 48 h | Acute toxicity test, histopathology | Fatty acids, lupeol, and terpenes | [114] | |
| Glass Petri dish | 6/group | adult | 96 h | Fish acute toxicity | Alkaloids, phenol, and flavonoids with some chemical groups of alkanes, aromatics, hydroxyls, carbonyls, aldehyde, amines, nitriles, amides and ethers | [78] |
| 30/Petri dish | 6 hpf | 72 h | Embryotoxicity | Moringa oleifera seed oil: 2,2-Bipydidine,3,3-diol, myristoleic acid, palmitoleic acid, stearic acid, oleic acid, arachidic acid, methyl-9-octadecanoate, adrenic acid, erucic acid, behenic acid, decamethylcyclopentasiloxane, α-tocopherol, γ-tocopherol, β-sitosterol, camphenone, hexahydrofarnesylacetone, linoleic acid, gadoleic/eicosenoic acid Moringa peregrina seed oil: palmitic acid, stearic acid, oleic acid, arachidic acid, gadoleic/eicosenoic acid, tricosane, erucic acid, behenic acid, α-tocopherol or γ-tocopherol, α-sitosterol, δ-tocopherol, 2-allyl-5-t-butylhydroquinone, isamoxole, hexahydrofarnesylacetone, linoleic acid, and β-sitosterol | [103] | |
| n.a. | 8-cell stage | 5 dpf | Embryotoxicity, teratogenic effects | 1,2-cyclopentanedione, 2,3-dihydro-3,5-dihydroxy-6-methyl-4h-pyran-4-one, 1,3;2,5-dimethylene-l-rhamnitol, elemol, (-)-selina-4.alpha.,11-diol, and beta-eudesmol | [100] | |
| 20/Petri dish | 1–4 cell stage | 72 h | Embryotoxicity | Shikonin, β,β-dimethylacrylalkannin, and β,β-dimethylacryl shikonin | [106] | |
| Rectangular glass tank | 12/group | n.a. | 48 h | Acute toxicity | Fatty acids, esters, alcohols, and the sterol sitosterol | [114] |
| REKO glass (artificial egg environment) | 20 fertilized eggs | 4 hpf | 48 h | Embryotoxicity, teratogenic effects | Phenolic compounds | [94] |
| 10/glass tank | n.a. | 48 h | Evaluation of genotoxicity and acute toxicity | Phenolic compounds, alkaloids, saponins, terpenoids, carbohydrates, and tannins | [10] | |
| Vial | 4/group (per well) | n.a. | 48–72 h | Cytotoxicity, toxicity, teratogenicity (hatchability and heartbeat rate) | n.a. | [87] |
| Vessel | 10/group | adult | 72 h | Fish acute toxicity | n.a. | [113] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modarresi Chahardehi, A.; Arsad, H.; Lim, V. Zebrafish as a Successful Animal Model for Screening Toxicity of Medicinal Plants. Plants 2020, 9, 1345. https://doi.org/10.3390/plants9101345
Modarresi Chahardehi A, Arsad H, Lim V. Zebrafish as a Successful Animal Model for Screening Toxicity of Medicinal Plants. Plants. 2020; 9(10):1345. https://doi.org/10.3390/plants9101345
Chicago/Turabian StyleModarresi Chahardehi, Amir, Hasni Arsad, and Vuanghao Lim. 2020. "Zebrafish as a Successful Animal Model for Screening Toxicity of Medicinal Plants" Plants 9, no. 10: 1345. https://doi.org/10.3390/plants9101345