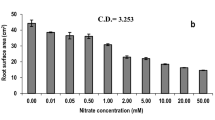
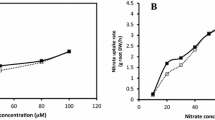
Abstract
Tissue and canopy-level evidence suggests that elevated carbon dioxide (EC) inhibits shoot nitrate assimilation in plants and thereby affects nitrogen (N) and protein content of the economic produce. It is speculated that species or genotypes relying more on root nitrate assimilation can adapt better under EC due to the improved/steady supply of reductants required for nitrate assimilation. A study was conducted to examine the effect of EC on N assimilation and associated gene expression in wheat seedlings. Wheat genotypes, BT-Schomburgk (BTS) with comparatively high leaf nitrate reductase (NR) activity and Gluyas Early (GE) with high root NR activity were grown in hydroponic culture for 30 days with two different nitrate levels (0.05 mM and 5 mM) in the climate controlled growth chambers maintained at either ambient (400 ± 10 μmol mol−1) or EC (700 ± 10 μmol mol−1) conditions. Exposure to EC downregulated the activity of enzyme NR and glutamate synthase (GOGAT) in leaf tissues, whereas in roots, activities of both the enzymes were upregulated by exposure to EC. In addition, EC downregulated N assimilation and signalling gene expression under high N availability. Root N assimilation was less affected in comparison with shoot N assimilation; thereby, the proportion of root contribution towards total assimilation was higher. The results suggest that EC could alter and re-programme N assimilation and signalling in wheat seedlings. The genotype and tissue-specific effects of EC on N assimilation also warrants the need for identification of suitable genotypes and revision of fertiliser regime for tapping the beneficial effects of EC conditions.






Similar content being viewed by others
References
Adavi SB, Sathee L (2019) Elevated CO2-induced production of nitric oxide differentially modulates nitrate assimilation and root growth of wheat seedlings in a nitrate dose-dependent manner. Protoplasma 256:147–159
Agüera E, Ruano D, Cabello P, de la Haba P (2006) Impact of atmospheric CO2 on growth, photosynthesis and nitrogen metabolism in cucumber (Cucumis sativus L.) plants. J Plant Physiol 163:809–817
Amthor JS (2001) Effects of atmospheric CO2 concentration on wheat yield: review of results from experiments using various approaches to control CO2 concentration. F Crop Res 73:1–34
Arp WJ, Drake BG (1991) Increased photosynthetic capacity of Scirpus olneyi after 4 years of exposure to elevated CO2. Plant Cell Environ 14:1003–1006
Backhausen JE, Emmerlich A, Holtgrefe S, Horton P, Nast G, Rogers JJM, Müller-Röber B, Scheibe R (1998) Transgenic potato plants with altered expression levels of chloroplast NADP-malate dehydrogenase: interactions between photosynthetic electron transport and malate metabolism in leaves and in isolated intact chloroplasts. Planta 207:105–114
Beidler KV, Taylor BN, Strand AE, Cooper ER, Schönholz M, Pritchard SG (2015) Changes in root architecture under elevated concentrations of CO2 and nitrogen reflect alternate soil exploration strategies. New Phytol 205:1153–1163
Benlloch-Gonzalez M, Berger J, Bramley H, Rebetzke G, Palta JA (2014) The plasticity of the growth and proliferation of wheat root system under elevated CO2. Plant Soil 374:963–976
Bloom AJ (2015) Photorespiration and nitrate assimilation: a major intersection between plant carbon and nitrogen. Photosynth Res 123:117–128
Bloom AJ, Smart DR, Nguyen DT, Searles PS (2002) Nitrogen assimilation and growth of wheat under elevated carbon dioxide. Proc Natl Acad Sci 99:1730–1735
Bloom AJ, Burger M, Asensio JSR, Cousins AB (2010) Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science (80- ) 328:899–903
Bloom AJ, Burger M, Kimball BA, Pinter PJ Jr (2014) Nitrate assimilation is inhibited by elevated CO2 in field-grown wheat. Nat Clim Chang 4:477–480
Buchner P, Tausz M, Ford R, Leo A, Fitzgerald GJ, Hawkesford MJ, Tausz-Posch S (2015) Expression patterns of C-and N-metabolism related genes in wheat are changed during senescence under elevated CO2 in dry-land agriculture. Plant Sci 236:239–249
Bunce JA (2002) Carbon dioxide concentration at night affects translocation from soybean leaves. Ann Bot 90:399–403
Conroy J, Hocking P (1993) Nitrogen nutrition of C3 plants at elevated atmospheric CO2 concentrations. Physiol Plant 89:570–576
de Mothes MA, Knoppik D (1994) Effects of long-term enhanced CO2 partial pressure on gas exchange parameters and saccharide pools in wheat leaves. Photosynth (Czech Republic) 30(3):435–445
Dong J, Li X, Chu W, Duan Z (2017) High nitrate supply promotes nitrate assimilation and alleviates photosynthetic acclimation of cucumber plants under elevated CO2. Sci Hortic (Amsterdam) 218:275–283
Downes MT (1978) An improved hydrazine reduction method for the automated determination of low nitrate levels in freshwater. Water Res 12:673–675
Foyer CH, Noctor G, Hodges M (2011) Respiration and nitrogen assimilation: targeting mitochondria-associated metabolism as a means to enhance nitrogen use efficiency. J Exp Bot 62:1467–1482
Geiger M, Walch-Liu P, Engels C, Harnecker J, Schulze ED, Ludewig F, Sonnewald U, Scheible WR, Stitt M (1998) Enhanced carbon dioxide leads to a modified diurnal rhythm of nitrate reductase activity in older plants, and a large stimulation of nitrate reductase activity and higher levels of amino acids in young tobacco plants. Plant Cell Environ 21:253–268
Geiger M, Haake V, Ludewig F, Sonnewald U, Stitt M (1999) The nitrate and ammonium nitrate supply have a major influence on the response of photosynthesis, carbon metabolism, nitrogen metabolism and growth to elevated carbon dioxide in tobacco. Plant Cell Environ 22:1177–1199
Grimmer C, Komor E (1999) Assimilate export by leaves of Ricinus communis L. growing under normal and elevated carbon dioxide concentrations: the same rate during the day, a different rate at night. Planta 209:275–281
Hachiya T, Sugiura D, Kojima M, Sato S, Yanagisawa S, Sakakibara H, Terashima I, Noguchi K (2014) High CO2 triggers preferential root growth of Arabidopsis thaliana via two distinct systems under low pH and low N stresses. Plant Cell Physiol 55:269–280
Hawkesford M, Horst W, Kichey T et al (2012) Functions of macronutrients. In: Marschner’s mineral nutrition of higher plants. pp. 135–189
He X, Qu B, Li W, Zhao X, Teng W, Ma W, Ren Y, Li B, Li Z, Tong Y (2015) The nitrate-inducible NAC transcription factor TaNAC2-5A controls nitrate response and increases wheat yield. Plant Physiol 169:1991–2005. https://doi.org/10.1104/pp.15.00568
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334
Ho C-H, Lin S-H, Hu H-C, Tsay Y-F (2009) CHL1 functions as a nitrate sensor in plants. Cell 138:1184–1194
Hocking PJ, Meyer CP (1991) Carbon dioxide enrichment decreases critical nitrate and nitrogen concentrations in wheat. J Plant Nutr 14:571–584
Igamberdiev AU, Bykova NV, Lea PJ, Gardeström P (2001) The role of photorespiration in redox and energy balance of photosynthetic plant cells: a study with a barley mutant deficient in glycine decarboxylase. Physiol Plant 111:427–438
Iversen CM (2010) Digging deeper: fine-root responses to rising atmospheric CO2 concentration in forested ecosystems. New Phytol 186:346–357
Jackson RB, Reynolds HL (1996) Nitrate and ammonium uptake for single-and mixed-species communities grown at elevated CO2. Oecologia 105:74–80
Jauregui I, Aparicio-Tejo PM, Avila C, Cañas R, Sakalauskiene S, Aranjuelo I (2016) Root--shoot interactions explain the reduction of leaf mineral content in Arabidopsis plants grown under elevated [CO2] conditions. Physiol Plant 158:65–79
Jayawardena DM, Heckathorn SA, Bista DR, Mishra S, Boldt JK, Krause CR (2017) Elevated CO2 plus chronic warming reduce nitrogen uptake and levels or activities of nitrogen-uptake and-assimilatory proteins in tomato roots. Physiol Plant 159:354–365
Jones JB (1987) Kjeldahl nitrogen determination—what’s in a name. J Plant Nutr 10(9–16):1675–1682
Jin Z, Zhu Y, Li X, Dong Y, An Z (2015) Soil N retention and nitrate leaching in three types of dunes in the Mu Us desert of China. Sci Rep 5:14222
Kimball BA, Morris CF, Pinter PJ et al (2001) Elevated CO2, drought and soil nitrogen effects on wheat grain quality. New Phytol 150:295–303
Klepper L, Flesher D, Hageman RH (1971) Generation of reduced nicotinamide adenine dinucleotide for nitrate reduction in green leaves. Plant Physiol 48:580–590
Kruse J, Hetzger I, Hänsch R, Mendel RR, Walch-Liu P, Engels C, Rennenberg H (2002) Elevated p CO2 favours nitrate reduction in the roots of wild-type tobacco (Nicotiana tabacum cv. Gat.) and significantly alters N-metabolism in transformants lacking functional nitrate reductase in the roots. J Exp Bot 53:2351–2367
la Mata L, la Haba P, Alamillo JM et al (2013) Elevated CO2 concentrations alter nitrogen metabolism and accelerate senescence in sunflower (Helianthus annuus L.) plants. Plant. Soil Environ 59:303–308
Larios B, Agüera E, Cabello P et al (2004) The rate of CO2 assimilation controls the expression and activity of glutamine synthetase through sugar formation in sunflower (Helianthus annuus L.) leaves. J Exp Bot 55:69–75
Lekshmy S, Vanita J, Sangeeta K et al (2009) Effect of elevated carbon dioxide on kinetics of nitrate uptake in wheat roots. Indian J Plant Physiol 14:16–22
Lekshmy S, Jain V, Khetarpal S et al (2016) Influence of elevated carbon dioxide and ammonium nutrition on growth and nitrogen metabolism in wheat (Triticum aestivum). Indian J Agric Sci 86:25–30
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2- ΔΔCT method. Methods 25:402–408
Mahmoud D, Pandey R, Sathee L, et al (2020) Regulation of expression of genes associated with nitrate response by osmotic stress and combined osmotic and nitrogen deficiency stress in bread wheat (Triticum aestivum L.). Plant Physiol Reports 1–16
Mariscal V, Moulin P, Orsel M, Miller AJ, Fernández E, Galván A (2006) Differential regulation of the Chlamydomonas Nar1 gene family by carbon and nitrogen. Protist 157:421–433
Martin T, Oswald O, Graham IA (2002) Arabidopsis seedling growth, storage lipid mobilization, and photosynthetic gene expression are regulated by carbon: nitrogen availability. Plant Physiol 128:472–481
McNally SF, Hirel B, Gadal P et al (1983) Glutamine synthetases of higher plants: evidence for a specific isoform content related to their possible physiological role and their compartmentation within the leaf. Plant Physiol 72:22–25
Miller AJ, Fan X, Shen Q, Smith SJ (2008) Amino acids and nitrate as signals for the regulation of nitrogen acquisition. J Exp Bot 59:111–119
Mohanty B, Fletcher JS (1980) Ammonium influence on nitrogen assimilating enzymes and protein accumulation in suspension cultures of Paul’s Scarlet rose. Physiol Plant 48:453–459
Nair TVR, Abrol YP (1973) Nitrate reductase activity in developing wheat ears. Experientia 29:1480–1481
Nie GY, Long SP, Garcia RL et al (1995) Effects of free-air CO2 enrichment on the development of the photosynthetic apparatus in wheat, as indicated by changes in leaf proteins. Plant Cell Environ 18:855–864
O’Brien JA, Vega A, Bouguyon E et al (2016) Nitrate transport, sensing, and responses in plants. Mol Plant 9:837–856
Padhan BK, Sathee L, Meena HS, Adavi SB (2020) CO2 elevation accelerates phenology and alters carbon / nitrogen metabolism vis-à-vis ROS abundance in bread wheat. Front Plant Sci 11:1–18. https://doi.org/10.3389/fpls.2020.01061
Pleijel H, Uddling J (2011) Yield vs. quality trade-offs for wheat in response to carbon dioxide and ozone. Glob Chang Biol 18:596–605
Porter JR, Xie L, Challinor AJ, Cochrane K, Howden SM, Iqbal MM, Lobell DB, Travasso MI (2014) Food security and food production systems. In: Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Field CB, VR Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC, Girma B, Kissel ES, Levy AN, MacCracken S, Mastrandrea PR, L.L.White LL (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA: 485–533
Qaderi MM, Reid DM (2008) Combined effects of temperature and carbon dioxide on plant growth and subsequent seed germinability of Silene noctiflora. Int J Plant Sci 169:1200–1209
Quesada A, Fernández E et al (2000) Involvement of chloroplast and mitochondria redox valves in nitrate assimilation. Trends Plant Sci 5:463–464
Rachmilevitch S, Cousins AB, Bloom AJ (2004) Nitrate assimilation in plant shoots depends on photorespiration. Proc Natl Acad Sci 101:11506–11510
Rahayu YS, Walch-Liu P, Neumann G, Römheld V, von Wirén N, Bangerth F (2005) Root-derived cytokinins as long-distance signals for NO3--induced stimulation of leaf growth. J Exp Bot 56:1143–1152
Raun WR, Johnson GV (1999) Improving nitrogen use efficiency for cereal production. Agron J 91:357–363
Schroeder PD, Radcliffe DE, Cabrera ML (2004) Rainfall timing and poultry litter application rate effects on phosphorus loss in surface runoff. J Environ Qual 33:2201–2209
Shimono H, Bunce JA (2009) Acclimation of nitrogen uptake capacity of rice to elevated atmospheric CO2 concentration. Ann Bot 103:87–94
Stitt M, Krapp A (1999) The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant Cell Environ 22:583–621
Taub DR, Wang X (2008) Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. J Integr Plant Biol 50:1365–1374
Tuba Z, Szente K, Koch J (1994) Response of photosynthesis, stomatal conductance, water use efficiency and production to long-term elevated CO2 in winter wheat. J Plant Physiol 144:661–668
Uprety DC, Dwivedi N, Jain V, Mohan R (2002) Effect of elevated carbon dioxide concentration on the stomatal parameters of rice cultivars. Photosynthetica 40:315–319
Vicente R, Pérez P, Martínez-Carrasco R, Feil R, Lunn JE, Watanabe M, Arrivault S, Stitt M, Hoefgen R, Morcuende R (2016) Metabolic and transcriptional analysis of durum wheat responses to elevated CO2 at low and high nitrate supply. Plant Cell Physiol 57:2133–2146. https://doi.org/10.1093/pcp/pcw131
Walch-Liu P, Neumann G, Bangerth F, Engels C (2000) Rapid effects of nitrogen form on leaf morphogenesis in tobacco. J Exp Bot 51:227–237
Xu G, Fan X, Miller AJ (2012) Plant nitrogen assimilation and use efficiency. Annu Rev Plant Biol 63:153–182
Yang T, Hao L, Yao S, Zhao Y, Lu W, Xiao K (2016) TabHLH1, a bHLHtype transcription factor gene in wheat, improves plant tolerance to P and N deprivation via regulation of nutrient transporter gene transcription and ROS homeostasis. Plant Physiol Biochem 104:99–113
Zerihun A, Gutschick VP, Bassirirad H (2000) Compensatory roles of nitrogen uptake and photosynthetic N-use efficiency in determining plant growth response to elevated CO2: evaluation using a functional balance model. Ann Bot 86:723–730
Acknowledgements
The authors are thankful to the ICAR-Indian Agricultural Research Institute for providing the necessary facilities.
Funding
This work was financially supported by the ICAR-Indian Agricultural Research Institute for funding (institute project-CRSCIARISIL20144047279). SAB was a recipient of ICAR-Junior research fellowship support, during the course of the study.
Author information
Authors and Affiliations
Contributions
SAB and LS conducted the experiments. LS finalised the experiments, designed primers and did the expression profiling and statistical analysis. LS and SAB together prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Handling Editor: Néstor Carrillo
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 4450 kb)
Rights and permissions
About this article
Cite this article
Adavi, S.B., Sathee, L. Elevated CO2 alters tissue balance of nitrogen metabolism and downregulates nitrogen assimilation and signalling gene expression in wheat seedlings receiving high nitrate supply. Protoplasma 258, 219–233 (2021). https://doi.org/10.1007/s00709-020-01564-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-020-01564-3