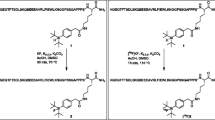
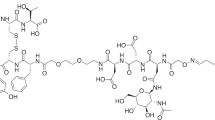
Abstract
There is an ongoing interest of having a somatostatin analog radiotracer labeled with 18F for PET imaging of neuroendocrine tumors. Nonetheless, there is none yet approved for clinical use. 18F is the ideal radionuclide for PET imaging due to its high β+ decay, suitable half-life and ease of production. As with other peptides, the labeling of SSTA with 18F comes along with its complications. In this review, we discuss the methods to label SSTA with 18F highlighting the chemistry as well as an overview in the progress on preclinical and clinical applications of 18F-labeled SSTA tracers.















Similar content being viewed by others
References
Oronsky B, Ma PC, Morgensztern D, Carter CA (2017) Nothing but NET: a review of neuroendocrine tumors and carcinomas. Neoplasia 19:991–1002. https://doi.org/10.1016/j.neo.2017.09.002
Öberg K, Sundin A (2016) In: Buchfelder M, Guaraldi F (eds). Karger https://doi.org/10.1159/000442331
Gore AC (2013) In: Squire LR, Berg D, Bloom FE, du Lac S, Ghosh A, Spitzer NC (eds) Fundamental neuroscience 4th edn. Academic Press, San Diego. https://doi.org/10.1016/B978-0-12-385870-2.00038-X
Colquhoun SD (2018) Neuroendocrine tumors with hepatic metastases: A review of evolving treatment options. Liver Res 2:92–99. https://doi.org/10.1016/j.livres.2018.08.002
Levine JE (2012) In: Fink G, Pfaff DW, Levine JE (eds) Handbook of neuroendocrinology. Academic Press, San Diego. https://doi.org/10.1016/B978-0-12-375097-6.10001-0
Merola E, Panzuto F, Delle Fave G (2017) Antiproliferative effect of somatostatin analogs in advanced gastro-entero-pancreatic neuroendocrine tumors: a systematic review and meta-analysis. Oncotarget 8:46624–46634. https://doi.org/10.18632/oncotarget.16686
Keskin O, Yalcin S (2013) A review of the use of somatostatin analogs in oncology. OncoTargets Ther 6:471–483. https://doi.org/10.2147/OTT.S39987
Modlin IM, Pavel M, Kidd M, Gustafsson BI (2010) Review article: somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine (carcinoid) tumours. Aliment Pharmacol Ther 31:169–188. https://doi.org/10.1111/j.1365-2036.2009.04174.x
Molitch ME (2012) In: Goldman L, Schafer AI (eds) Goldman's cecil medicine, 24th edn. W.B. Saunders, Philadelphia. https://doi.org/10.1016/B978-1-4377-1604-7.00230-X
Patel YC (1999) Somatostatin and its receptor family. Front Neuroendocrinol 20:157–198. https://doi.org/10.1006/frne.1999.0183
Dasgupta P (2004) Somatostatin analogues: multiple roles in cellular proliferation, neoplasia, and angiogenesis. Pharmacol Ther 102:61–85. https://doi.org/10.1016/j.pharmthera.2004.02.002
Johnbeck CB, Knigge U, Kjær A (2014) PET tracers for somatostatin receptor imaging of neuroendocrine tumors: current status and review of the literature. Future Oncol 10:2259–2277. https://doi.org/10.2217/fon.14.139
Kaltsas GA, Besser GM, Grossman AB (2004) The diagnosis and medical management of advanced neuroendocrine tumors. Endocr Rev 25:458–511. https://doi.org/10.1210/er.2003-0014
Smit Duijzentkunst DA, Kwekkeboom DJ, Bodei L (2017) Somatostatin receptor 2–targeting compounds. J Nucl Med 58:54S–60S. https://doi.org/10.2967/jnumed.117.191015
Mizutani G, Nakanishi Y, Watanabe N, Honma T, Obana Y, Seki T, Ohni S, Nemoto N (2012) Expression of somatostatin receptor (SSTR) subtypes (SSTR-1, 2A, 3, 4 and 5) in neuroendocrine tumors using real-time RT-PCR method and immunohistochemistry. Acta Histochem Cytochem 45:167–176. https://doi.org/10.1267/ahc.12006
Bauer W, Briner U, Doepfner W, Haller R, Huguenin R, Marbach P, Petcher TJ, Pless J (1982) SMS 201–995: a very potent and selective octapeptide analogue of somatostatin with prolonged action. Life Sci 31:1133–1140. https://doi.org/10.1016/0024-3205(82)90087-X
Marciniak A, Brasuń J (2017) Somatostatin analogues labeled with copper radioisotopes: current status. J Radioanal Nucl Chem 313:279–289. https://doi.org/10.1007/s10967-017-5323-x
Reubi JC, Schär J-C, Waser B, Wenger S, Heppeler A, Schmitt JS, Mäcke HR (2000) Affinity profiles for human somatostatin receptor subtypes SST1–SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med Mol Imaging 27:273–282. https://doi.org/10.1007/s002590050034
Peng Y, Deng L, Ding Y, Chen Q, Wu Y, Yang M, Wang Y, Fu Q (2014) Comparative study of somatostatin-human serum albumin fusion proteins and natural somatostatin on receptor binding, Internalization and Activation. PLoS ONE 9:e89932. https://doi.org/10.1371/journal.pone.0089932
Pauwels E, Cleeren F, Bormans G, Deroose CM (2018) Somatostatin receptor PET ligands—the next generation for clinical practice. Am J Nucl Med Mol Imaging 8:311–331
Antunes P, Ginj M, Zhang H, Waser B, Baum RP, Reubi JC, Maecke H (2007) Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur J Nucl Med Mol Imaging 34:982–993. https://doi.org/10.1007/s00259-006-0317-x
Richter S, Wuest F (2014) 18F-labeled peptides: the future is bright. Molecules 19:20536–20556. https://doi.org/10.3390/molecules191220536
Krenning EP, Breeman WAP, Kooij PPM, Lameris JS, Bakker WH, Koper JW, Ausema L, Reubi JC, Lamberts SWJ (1989) Localisation of endocrine-related tumours with radioiodinated analogue of somatostatin. Lancet 333:242–244. https://doi.org/10.1016/S0140-6736(89)91258-0
Krenning EP, Kwekkeboom DJ, Bakker WH, Breeman WAP, Kooij PPM, Oei HY, van Hagen M, Postema PTE, de Jong M, Reubi JC, Visser TJ, Reijs AEM, Hofland LJ, Koper JW, Lamberts SWJ (1993) Somatostatin receptor scintigraphy with [111In-DTPA-d-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med Mol Imaging 20:716–731. https://doi.org/10.1007/BF00181765
Waldmann CM, Stuparu AD, van Dam RM, Slavik R (2019) The Search for an alternative to [68Ga]Ga-DOTA-TATE in neuroendocrine tumor theranostics: current state of 18F-labeled somatostatin analog development. Theranostics 9:1336–1347. https://doi.org/10.7150/thno.31806
Virgolini I, Ambrosini V, Bomanji JB, Baum RP, Fanti S, Gabriel M, Papathanasiou ND, Pepe G, Oyen W, De Cristoforo C, Chiti A (2010) Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE. Eur J Nucl Med Mol Imaging 37:2004–2010. https://doi.org/10.1007/s00259-010-1512-3
Werner RA, Bluemel C, Allen-Auerbach MS, Higuchi T, Herrmann K (2015) 68Gallium- and 90Yttrium-/ 177Lutetium: "theranostic twins" for diagnosis and treatment of NETs. Ann Nucl Med 29:1–7. https://doi.org/10.1007/s12149-014-0898-6
Norenberg JP, Krenning BJ, Konings IRHM, Kusewitt DF, Nayak TK, Anderson TL, de Jong M, Garmestani K, Brechbiel MW, Kvols LK (2006) 213Bi-[DOTA0, Tyr3]Octreotide peptide receptor radionuclide therapy of pancreatic tumors in a preclinical animal model. Clin Cancer Res 12:897–903. https://doi.org/10.1158/1078-0432.ccr-05-1264
Miederer M, Henriksen G, Alke A, Mossbrugger I, Quintanilla-Martinez L, Senekowitsch-Schmidtke R, Essler M (2008) Preclinical evaluation of the α-particle generator nuclide 225Ac for somatostatin receptor radiotherapy of neuroendocrine tumors. Clin Cancer Res 14:3555–3561. https://doi.org/10.1158/1078-0432.ccr-07-4647
Hennrich U, Benešová M (2020) [68Ga]Ga-DOTA-TOC: the first FDA-approved 68Ga-radiopharmaceutical for PET imaging. Pharmaceuticals. https://doi.org/10.3390/ph13030038
Rösch F (2013) Past, present and future of 68Ge/68Ga generators. Appl Radiat Isot 76:24–30. https://doi.org/10.1016/j.apradiso.2012.10.012
Alves F, Alves VHP, Carmo SJCD, Neves ACB, Silva M, Abrunhosa AJ (2017) Production of copper-64 and gallium-68 with a medical cyclotron using liquid targets. Mod Phys Lett A 32:1740013. https://doi.org/10.1142/s0217732317400132
Lin M, Waligorski GJ, Lepera CG (2018) Production of curie quantities of 68Ga with a medical cyclotron via the 68Zn(p, n)68Ga reaction. Appl Radiat Isot 133:1–3. https://doi.org/10.1016/j.apradiso.2017.12.010
Nelson BJB, Wilson J, Richter S, Duke MJM, Wuest M, Wuest F (2020) Taking cyclotron 68Ga production to the next level: expeditious solid target production of 68Ga for preparation of radiotracers. Nucl Med Biol 80–81:24–31. https://doi.org/10.1016/j.nucmedbio.2020.01.005
Johnbeck C, Knigge U, Loft A, Berthelsen A, Mortensen J, Oturai P, Langer S, Elema D, Kjaer A (2016) Head to Head comparison of 64Cu-DOTATATE and 68Ga-DOTATOC PET/CT: a prospective study of 59 patients with neuroendocrine tumors. J Nucl Med 57:151
Kjaer A, Binderup T, Johnbeck C, Carlsen E, Langer S, Federspiel B, Knigge U (2019) 64Cu-DOTATATE somatostatin receptor imaging in neuroendocrine tumors: experience from 500 patients at Copenhagen ENETS center of excellence. J Nucl Med 60:504
Avila-Rodriguez MA, Nye JA, Nickles RJ (2007) Simultaneous production of high specific activity 64Cu and 61Co with 11.4 MeV protons on enriched 64Ni nuclei. Appl Radiat Isot 65:1115–1120. https://doi.org/10.1016/j.apradiso.2007.05.012
McCarthy DW, Shefer RE, Klinkowstein RE, Bass LA, Margeneau WH, Cutler CS, Anderson CJ, Welch MJ (1997) Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl Med Biol 24:35–43. https://doi.org/10.1016/S0969-8051(96)00157-6
Jacobson O, Kiesewetter DO, Chen X (2015) Fluorine-18 radiochemistry, labeling strategies and synthetic routes. Bioconjug Chem 26:1–18. https://doi.org/10.1021/bc500475e
Shuanglong L, Bin S, Frederick TC, Zhen C (2011) Recent progress in radiofluorination of peptides for PET molecular imaging. Curr Org Synth 8:584–592. https://doi.org/10.2174/157017911796117197
Jamous M, Haberkorn U, Mier W (2013) Synthesis of peptide radiopharmaceuticals for the therapy and diagnosis of tumor diseases. Molecules 18:3379–3409. https://doi.org/10.3390/molecules18033379
Reddy VP (2015) In: Reddy VP (ed) Organofluorine compounds in biology and medicine. Elsevier, Amsterdam. https://doi.org/10.1016/B978-0-444-53748-5.00007-1
Kapty J, Kniess T, Wuest F, Mercer JR (2011) Radiolabeling of phosphatidylserine-binding peptides with prosthetic groups N-[6-(4-[18F]fluorobenzylidene)aminooxyhexyl]maleimide ([18F]FBAM) and N-succinimidyl-4-[18F]fluorobenzoate ([18F]SFB). Appl Radiat Isot 69:1218–1225. https://doi.org/10.1016/j.apradiso.2011.05.012
Kniess T, Laube M, Brust P, Steinbach J (2015) 2-[18F]Fluoroethyl tosylate—a versatile tool for building 18F-based radiotracers for positron emission tomography. MedChemComm 6:1714–1754. https://doi.org/10.1039/C5MD00303B
Vaidyanathan G, Zalutsky MR (2006) Synthesis of N-succinimidyl 4-[18F]fluorobenzoate, an agent for labeling proteins and peptides with 18F. Nat Protoc 1:1655–1661. https://doi.org/10.1038/nprot.2006.264
Wuest F, Köhler L, Berndt M, Pietzsch J (2008) Systematic comparison of two novel, thiol-reactive prosthetic groups for 18F labeling of peptides and proteins with the acylation agent succinimidyl-4-[18F]fluorobenzoate ([18F]SFB). Amino Acids 36:283. https://doi.org/10.1007/s00726-008-0065-2
Jacobson O, Zhu L, Ma Y, Weiss ID, Sun X, Niu G, Kiesewetter DO, Chen X (2011) Rapid and simple one-step F-18 labeling of peptides. Bioconjug Chem 22:422–428. https://doi.org/10.1021/bc100437q
Schirrmacher R, Bradtmöller G, Schirrmacher E, Thews O, Tillmanns J, Siessmeier T, Buchholz HG, Bartenstein P, Wängler B, Niemeyer CM, Jurkschat K (2006) 18F-Labeling of peptides by means of an organosilicon-based fluoride acceptor. Angew Chem Int Ed 45:6047–6050. https://doi.org/10.1002/anie.200600795
Laverman P, McBride WJ, Sharkey RM, Eek A, Joosten L, Oyen WJG, Goldenberg DM, Boerman OC (2010) A novel facile method of labeling octreotide with 18F-fluorine. J Nucl Med 51:454–461. https://doi.org/10.2967/jnumed.109.066902
McBride WJ, Sharkey RM, Karacay H, D'Souza CA, Rossi EA, Laverman P, Chang C-H, Boerman OC, Goldenberg DM (2009) A novel method of 18F radiolabeling for PET. J Nucl Med 50:991–998. https://doi.org/10.2967/jnumed.108.060418
Guhlke S, Wester H-J, Bruns C, Stöcklin G (1994) (2-[18F]fluoropropionyl-(d)phe1)-octreotide, a potential radiopharmaceutical for quantitative somatostatin receptor imaging with PET: synthesis, radiolabeling, in vitro validation and biodistribution in mice. Nucl Med Biol 21:819–825. https://doi.org/10.1016/0969-8051(94)90161-9
Iddon L, Leyton J, Indrevoll B, Glaser M, Robins EG, George AJT, Cuthbertson A, Luthra SK, Aboagye EO (2011) Synthesis and in vitro evaluation of [18F]fluoroethyl triazole labelled [Tyr3]octreotate analogues using click chemistry. Bioorg Med Chem Lett 21:3122–3127. https://doi.org/10.1016/j.bmcl.2011.03.016
Maschauer S, Heilmann M, Wängler C, Schirrmacher R, Prante O (2016) Radiosynthesis and preclinical evaluation of 18F-fluoroglycosylated octreotate for somatostatin receptor imaging. Bioconjug Chem 27:2707–2714. https://doi.org/10.1021/acs.bioconjchem.6b00472
Panico M, Lang L, Sassaman MB, Eckelman WC (2001) Radiolabeling of unprotected octreotide with F-18. J Label Compd Radiopharm 44:S922–S924. https://doi.org/10.1002/jlcr.25804401324
Schottelius M, Poethko T, Herz M, Reubi J-C, Kessler H, Schwaiger M, Wester H-J (2004) First 18F-labeled tracer suitable for routine clinical imaging of SST receptor-expressing tumors using positron emission tomography. Clin Cancer Res 10:3593. https://doi.org/10.1158/1078-0432.CCR-03-0359
Wester H, Schottelius M, Scheidhauer K, Meisetschläger G, Herz M, Rau F, Reubi J, Schwaiger M (2003) PET imaging of somatostatin receptors: design, synthesis and preclinical evaluation of a novel 18F-labelled, carbohydrated analogue of octreotide. Eur J Nucl Med Mol Imaging 30:117–122. https://doi.org/10.1007/s00259-002-1012-1
Lisova K, Sergeev M, Evans-Axelsson S, Stuparu AD, Beykan S, Collins J, Jones J, Lassmann M, Herrmann K, Perrin D, Lee JT, Slavik R, van Dam RM (2018) Microscale radiosynthesis, preclinical imaging and dosimetry study of [18F]AMBF3-TATE: a potential PET tracer for clinical imaging of somatostatin receptors. Nucl Med Biol 61:36–44. https://doi.org/10.1016/j.nucmedbio.2018.04.001
Litau S, Niedermoser S, Vogler N, Roscher M, Schirrmacher R, Fricker G, Wängler B, Wängler C (2015) Next generation of SiFAlin-based TATE derivatives for PET imaging of SSTR-positive tumors: influence of molecular design on in vitro SSTR binding and in vivo pharmacokinetics. Bioconjug Chem 26:2350–2359. https://doi.org/10.1021/acs.bioconjchem.5b00510
Liu Z, Pourghiasian M, Bénard F, Pan J, Lin K-S, Perrin DM (2014) Preclinical evaluation of a high-affinity 18F-trifluoroborate octreotate derivative for somatostatin receptor imaging. J Nucl Med 55:1499–1505. https://doi.org/10.2967/jnumed.114.137836
Niedermoser S, Chin J, Wängler C, Kostikov A, Bernard-Gauthier V, Vogler N, Soucy J-P, McEwan AJ, Schirrmacher R, Wängler B (2015) In vivo evaluation of 18F-SiFAlin–modified TATE: a potential challenge for 68Ga-DOTATATE, the clinical gold standard for somatostatin receptor imaging with PET. J Nucl Med 56:1100–1105. https://doi.org/10.2967/jnumed.114.149583
Schirrmacher E, Wängler B, Cypryk M, Bradtmöller G, Schäfer M, Eisenhut M, Jurkschat K, Schirrmacher R (2007) Synthesis of p-(Di-tert-butyl[18F]fluorosilyl)benzaldehyde ([18F]SiFA-A) with high specific activity by isotopic exchange: a convenient labeling synthon for the 18F-labeling of N-amino-oxy derivatized peptides. Bioconjug Chem 18:2085–2089. https://doi.org/10.1021/bc700195y
Wängler C, Waser B, Alke A, Iovkova L, Buchholz H-G, Niedermoser S, Jurkschat K, Fottner C, Bartenstein P, Schirrmacher R, Reubi J-C, Wester H-J, Wängler B (2010) One-step 18F-labeling of carbohydrate-conjugated octreotate-derivatives containing a silicon-fluoride-acceptor (SiFA). In vitro and in vivo evaluation as tumor imaging agents for positron emission tomography (PET). Bioconjug Chem 21:2289–2296. https://doi.org/10.1021/bc100316c
Laverman P, D’Souza CA, Eek A, McBride WJ, Sharkey RM, Oyen WJG, Goldenberg DM, Boerman OC (2012) Optimized labeling of NOTA-conjugated octreotide with F-18. Tumor Biol 33:427–434. https://doi.org/10.1007/s13277-011-0250-x
Guhlke S, Coenen HH, Stöcklin G (1994) Fluoroacylation agents based on small n.c.a. [18F]fluorocarboxylic acids. Appl Radiat Isot 45:715–727. https://doi.org/10.1016/0969-8043(94)90252-6
Symposium abstracts continued in part 4 (1993). J Label Compd Radiopharm, vol 32, pp 99–146. https://doi.org/10.1002/jlcr.2580320104
Wester H-J, Brockmann J, Rösch F, Wutz W, Herzog H, Smith-Jones P, Stolz B, Bruns C, Stöcklin G (1997) PET-pharmacokinetics of 18F-octreotide: A comparison with 67Ga-DFO and 86Y-DTPA-octreotide. Nucl Med Biol 24:275–286. https://doi.org/10.1016/S0969-8051(97)00039-5
Wester H-J, Schottelius M, Scheidhauer K, Reubi J-C, Wolf I, Schwaiger M (2002) Comparison of radioiodinated TOC, TOCA and Mtr-TOCA: the effect of carbohydration on the pharmacokinetics. Eur J Nucl Med Mol Imaging 29:28–38. https://doi.org/10.1007/s00259-001-0669-1
Schottelius M, Wester H-J, Reubi JC, Senekowitsch-Schmidtke R, Schwaiger M (2002) Improvement of pharmacokinetics of radioiodinated Tyr3-octreotide by conjugation with carbohydrates. Bioconjug Chem 13:1021–1030. https://doi.org/10.1021/bc0200069
Meisetschläger G, Poethko T, Stahl A, Wolf I, Scheidhauer K, Schottelius M, Herz M, Wester HJ, Schwaiger M (2006) Gluc-Lys([18F]FP)-TOCA PET in patients with SSTR-positive tumors: biodistribution and diagnostic evaluation compared with [111In]DTPA-octreotide. J Nucl Med 47:566–573
Allott L, Barnes C, Brickute D, Aboagye EO (2019) An improved automated radiosynthesis of [18F]FET-βAG-TOCA. React Chem Eng 4:569–574. https://doi.org/10.1039/C8RE00279G
Dubash SR, Keat N, Mapelli P, Twyman F, Carroll L, Kozlowski K, Al-Nahhas A, Saleem A, Huiban M, Janisch R, Frilling A, Sharma R, Aboagye EO (2016) Clinical translation of a click-labeled 18F-octreotate radioligand for imaging neuroendocrine tumors. J Nucl Med 57:1207–1213. https://doi.org/10.2967/jnumed.115.169532
Leyton J, Iddon L, Perumal M, Indrevoll B, Glaser M, Robins E, George AJT, Cuthbertson A, Luthra SK, Aboagye EO (2011) Targeting somatostatin receptors: preclinical evaluation of novel 18F-fluoroethyltriazole-Tyr3-octreotate analogs for PET. J Nucl Med 52:1441–1448. https://doi.org/10.2967/jnumed.111.088906
Allott L, Dubash S, Aboagye EO (2020) [18F]FET-βAG-TOCA: the design, evaluation and clinical translation of a fluorinated octreotide. Cancers 12:865
Dubash SR, Barwick T, Mauri FA, Kozlowski K, Frilling A, Valle JW, Lamarca A, Sharma R, Aboagye E (2018) [18F]FET-βAG-TOCA versus [68Ga]DOTATATE PET/CT in functional imaging of neuroendocrine tumours. J Clin Oncol 36:e24193–e24193. https://doi.org/10.1200/JCO.2018.36.15_suppl.e24193
Bouvet VR, Wuest F (2013) Application of [18F]FDG in radiolabeling reactions using microfluidic technology. Lab Chip 13:4290–4294. https://doi.org/10.1039/C3LC50797A
Wuest F, Hultsch C, Berndt M, Bergmann R (2009) Direct labelling of peptides with 2-[18F]fluoro-2-deoxy-d-glucose ([18F]FDG). Bioorg Med Chem Lett 19:5426–5428. https://doi.org/10.1016/j.bmcl.2009.07.108
Bernard-Gauthier V, Wängler C, Schirrmacher E, Kostikov A, Jurkschat K, Wängler B, Schirrmacher R (2014) 18F-labeled silicon-based fluoride acceptors: potential opportunities for novel positron emitting radiopharmaceuticals. BioMed Res Int 2014:454503. https://doi.org/10.1155/2014/454503
Ilhan H, Lindner S, Todica A, Cyran CC, Tiling R, Auernhammer CJ, Spitzweg C, Boeck S, Unterrainer M, Gildehaus FJ, Böning G, Jurkschat K, Wängler C, Wängler B, Schirrmacher R, Bartenstein P (2020) Biodistribution and first clinical results of 18F-SiFAlin-TATE PET: a novel 18F-labeled somatostatin analog for imaging of neuroendocrine tumors. Eur J Nucl Med Mol Imaging 47:870–880. https://doi.org/10.1007/s00259-019-04501-6
Lindner S, Simmet M, Gildehaus FJ, Jurkschat K, Wängler C, Wängler B, Bartenstein P, Schirrmacher R, Ilhan H (2020) Automated production of [18F]SiTATE on a Scintomics GRP™ platform for PET/CT imaging of neuroendocrine tumors. Nucl Med Biol 88–89:86–95. https://doi.org/10.1016/j.nucmedbio.2020.07.008
Lau J, Pan J, Rousseau E, Uribe CF, Seelam SR, Sutherland BW, Perrin DM, Lin K-S, Bénard F (2020) Pharmacokinetics, radiation dosimetry, acute toxicity and automated synthesis of [18F]AMBF3-TATE. EJNMMI Res 10:25. https://doi.org/10.1186/s13550-020-0611-9
Fersing C, Bouhlel A, Cantelli C, Garrigue P, Lisowski V, Guillet B (2019) A comprehensive review of non-covalent radiofluorination approaches using aluminum [18F]fluoride: will [18F]AlF replace 68Ga for metal chelate labelling? Molecules 24:2866
Kumar K, Ghosh A (2018) 18F-AlF labeled peptide and protein conjugates as positron emission tomography imaging pharmaceuticals. Bioconjug Chem 29:953–975. https://doi.org/10.1021/acs.bioconjchem.7b00817
Kumar K (2018) 18F-AlF–labeled biomolecule conjugates as imaging pharmaceuticals. J Nucl Med 59:1208–1209. https://doi.org/10.2967/jnumed.118.210609
McBride WJ, Sharkey RM, Goldenberg DM (2013) Radiofluorination using aluminum-fluoride (Al18F). EJNMMI Res 3:36. https://doi.org/10.1186/2191-219X-3-36
Tshibangu T, Cawthorne C, Serdons K, Pauwels E, Gsell W, Bormans G, Deroose CM, Cleeren F (2020) Automated GMP compliant production of [18F]AlF-NOTA-octreotide. EJNMMI Radiopharm Chem 5:4–4. https://doi.org/10.1186/s41181-019-0084-1
Allott L, Da Pieve C, Turton DR, Smith G (2017) A general [18F]AlF radiochemistry procedure on two automated synthesis platforms. React Chem Eng 2:68–74. https://doi.org/10.1039/C6RE00204H
Long T, Yang N, Zhou M, Chen D, Li Y, Li J, Tang Y, Liu Z, Li Z, Hu S (2019) Clinical application of 18F-AlF-NOTA-octreotide PET/CT in combination With 18F-FDG PET/CT for imaging neuroendocrine neoplasms. Clin Nucl Med 44:452–458. https://doi.org/10.1097/rlu.0000000000002578
Pauwels E, Cleeren F, Tshibangu T, Koole M, Serdons K, Dekervel J, Van Cutsem E, Verslype C, Van Laere K, Bormans G, Deroose CM (2019) Al18F-NOTA-octreotide: first comparison with 68Ga-DOTATATE in a neuroendocrine tumour patient. Eur J Nucl Med Mol Imaging 46:2398–2399. https://doi.org/10.1007/s00259-019-04425-1
Pauwels E, Cleeren F, Tshibangu T, Koole M, Serdons K, Dekervel J, Van Cutsem E, Verslype C, Van Laere K, Bormans G, Deroose CM (2020) [18F]AlF-NOTA-octreotide PET imaging: biodistribution, dosimetry and first comparison with [68Ga]Ga-DOTATATE in neuroendocrine tumour patients. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-020-04918-4
Acknowledgements
This research was supported by UNAM-DGAPA Grant PAPIIT-IT202518 and CONACYT Grants APN-2017-4837 and LN-2018-293334. DJP received a Postdoctoral fellowship from Programa de Becas Posdoctorales-DGAPA-UNAM. The authors want to thank Mrs. Josefina Bolado, Head of the Scientific Paper Translation Department, from División de Investigación at Facultad de Medicina, UNAM, for editing the English-language version of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pérez, D.J., Ávila-Rodríguez, M.A. Methods to radiolabel somatostatin analogs with [18F]fluoride: current status, challenges, and progress in clinical applications. J Radioanal Nucl Chem 326, 1519–1542 (2020). https://doi.org/10.1007/s10967-020-07437-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07437-6