Abstract
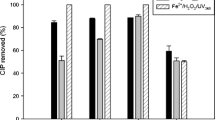
We used Low pressure mercury vapor lamp activated of Sodium Persulfate (UV/SPS) and Fenton processes in two separate reactors to comparison of cephalexin (CPX) degradation in aqueous solution. The effect of pH, initial concentration of SPS, concentration of CPX, concentration of H2O2 and concentration of Fe2+ on the degradation of CPX were investigated. The residue of CPX and metabolites were determined by HPLC and GC/MS. The Total Organic Carbon (TOC) analysis was utilized for surveying the mineralization of CPX. Biodegradability of CPX in both advanced oxidation processes was evaluated by BOD5/COD in optimum condition. The results indicated that the maximum CPX removal was obtained at pH 3, H2O2 3 mM, concentration of initial CPX 10 mg/L and by increasing the doses of SPS from 0.1 to 0.2 mM, the degradation of CPX was enhanced. In this study, the most important factors for AOP efficiency was concentration of initial CPX; and then pH in UV/SPS and H2O2 in Fenton processes. The TOC measurements indicate that the UV/SPS and Fenton can efficiently mineralize CPX. CPX removed enough to achieve suitable biodegradability for a further biological process. Too, analysis of generated intermediates during the degradation of CPX was conducted by GC/MS method and a degradation pathway was proposed.










Similar content being viewed by others
References
Coledam DA, et al. Electrochemical mineralization of cephalexin using a conductive diamond anode: a mechanistic and toxicity investigation. Chemosphere. 2017;168:638–47.
Le-Minh N, et al. Fate of antibiotics during municipal water recycling treatment processes. Water Res. 2010;44(15):4295–323.
Frontistis Z, Mantzavinos D, Meriç S. Degradation of antibiotic ampicillin on boron-doped diamond anode using the combined electrochemical oxidation-sodium persulfate process. J Environ Manag. 2018;223:878–87.
Almasi A, et al. Application of response surface methodology on cefixime removal from aqueous solution by ultrasonic/photooxidation. Int J Pharm Technol. 2016;8(3):16728–36.
Authority, E.F.S., E.C.f.D. Prevention, and Control. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2016. EFSA J. 2018;16(2):e05182.
Homem V, Santos L. Degradation and removal methods of antibiotics from aqueous matrices–a review. J Environ Manag. 2011;92(10):2304–47.
Moreira FC, Boaventura RAR, Brillas E, Vilar VJP. Electrochemical advanced oxidation processes: a review on their application to synthetic and real wastewaters. Appl Catal B Environ. 2017;202:217–61.
Oturan N, Ganiyu SO, Raffy S, Oturan MA. Sub-stoichiometric titanium oxide as a new anode material for electro-Fenton process: application to electrocatalytic destruction of antibiotic amoxicillin. Appl Catal B Environ. 2017;217:214–23.
Popescu M, Sandu C, Rosales E, Pazos M, Lazar G, Sanromán MÁ. Evaluation of different cathodes and reaction parameters on the enhancement of the electro-Fenton process. J Electroanal Chem. 2018;808:455–63.
Almasi A, et al. Efficiency of integrated ultrasonic and anaerobic digestion of oil refinery wastewater sludge. Glob NEST J. 2016;18(4):771–7.
Xiao R, Luo Z, Wei Z, Luo S, Spinney R, Yang W, et al. Activation of peroxymonosulfate/persulfate by nanomaterials for sulfate radical-based advanced oxidation technologies. Current Opinion Chem Eng. 2018;19:51–8.
Ghauch A, Baalbaki A, Amasha M, el Asmar R, Tantawi O. Contribution of persulfate in UV-254 nm activated systems for complete degradation of chloramphenicol antibiotic in water. Chem Eng J. 2017;317:1012–25.
Lata K, Sharma R, Naik L, Rajput YS, Mann B. Synthesis and application of cephalexin imprinted polymer for solid phase extraction in milk. Food Chem. 2015;184:176–82.
Ebrahiem EE, Al-Maghrabi MN, Mobarki AR. Removal of organic pollutants from industrial wastewater by applying photo-Fenton oxidation technology. Arab J Chem. 2017;10:S1674–9.
Lopez-Lopez C, Martín-Pascual J, Martínez-Toledo MV, Muñío MM, Hontoria E, Poyatos JM. Kinetic modelling of TOC removal by H2O2/UV, photo-Fenton and heterogeneous photocatalysis processes to treat dye-containing wastewater. Int J Environ Sci Technol. 2015;12(10):3255–62.
Bahrami Asl F, et al. Removal of metronidazole from aqueous solution using ozonation process. J Mazandaran Univ Med Sci. 2015;24(121):131–40.
Cai H, Liu X, Zou J, Xiao J, Yuan B, Li F, et al. Multi-wavelength spectrophotometric determination of hydrogen peroxide in water with peroxidase-catalyzed oxidation of ABTS. Chemosphere. 2018;193:833–9.
Gao Y-q, et al. Oxidation of the β-blocker propranolol by UV/persulfate: Effect, mechanism and toxicity investigation. Chemosphere. 2018;201:50–8.
Federation, W.E. and A.P.H. Association. Standard methods for the examination of water and wastewater. Washington: American Public Health Association (APHA); 2005.
Bansal P, Verma A. Synergistic effect of dual process (photocatalysis and photo-Fenton) for the degradation of cephalexin using TiO2 immobilized novel clay beads with waste fly ash/foundry sand. J Photochem Photobiol A Chem. 2017;342:131–42.
Almasi A, Mohammadi M, Shamsi K, Mohammadi S, Saeidimoghadam Z. Sonolytic and photocatalytic (sonophotocatalytic) removal of cephalexin from aqueous solution: process optimization using response surface methodology (RSM). Desalin Water Treat. 2017;85:256–63.
Babu, D.J., P. King, and Y.P. Kumar, Optimization of Cu (II) biosorption onto sea urchin test using response surface methodology and artificial neural networks. Int J Environ Sci Technol, 2018: p. 1–12.
O'Neill CM, Cruz-Romero MC, Duffy G, Kerry JP. The application of response surface methodology for the development of sensory accepted low-salt cooked ham using high pressure processing and a mix of organic acids. Innovative Food Sci Emerg Technol. 2018;45:401–11.
Villegas-Guzman P, Silva-Agredo J, Florez O, Giraldo-Aguirre AL, Pulgarin C, Torres-Palma RA. Selecting the best AOP for isoxazolyl penicillins degradation as a function of water characteristics: effects of pH, chemical nature of additives and pollutant concentration. J Environ Manag. 2017;190:72–9.
Zazouli MA, Taghavi M, Bazrafshan E. Influences of solution chemistry on phenol removal from aqueous environments by electrocoagulation process using aluminum electrodes. J Health Scope. 2012;1(2):66–70.
Miklos DB, Hartl R, Michel P, Linden KG, Drewes JE, Hübner U. UV/H2O2 process stability and pilot-scale validation for trace organic chemical removal from wastewater treatment plant effluents. Water Res. 2018;136:169–79.
Ajoudanian N, Nezamzadeh-Ejhieh A. Enhanced photocatalytic activity of nickel oxide supported on clinoptilolite nanoparticles for the photodegradation of aqueous cephalexin. Mater Sci Semicond Process. 2015;36:162–9.
Peiris C, Gunatilake SR, Mlsna TE, Mohan D, Vithanage M. Biochar based removal of antibiotic sulfonamides and tetracyclines in aquatic environments: a critical review. Bioresour Technol. 2017;246:150–9.
Lu J, Chen R, Liang H, Yan Q. The influence of concentration of hydroxyl radical on the chemical mechanical polishing of SiC wafer based on the Fenton reaction. Precis Eng. 2018;52:221–6.
Soltani RDC, et al. Implementation of martite nanoparticles prepared through planetary ball milling as a heterogeneous activator of oxone for degradation of tetracycline antibiotic: ultrasound and peroxy-enhancement. Chemosphere. 2018;210:699–708.
Wu S, Li Y, Zhao X, du Q, Wang Z, Xia Y, et al. Biosorption behavior of ciprofloxacin onto Enteromorpha prolifera: isotherm and kinetic studies. Int J Phytoremediation. 2015;17(10):957–61.
Choi Y-J, Kim L-H, Zoh K-D. Removal characteristics and mechanism of antibiotics using constructed wetlands. Ecol Eng. 2016;91:85–92.
Rayaroth MP, Aravind UK, Aravindakumar CT. Degradation of pharmaceuticals by ultrasound-based advanced oxidation process. Environ Chem Lett. 2016;14(3):259–90.
Wu J, Zhang H, Qiu J. Degradation of acid Orange 7 in aqueous solution by a novel electro/Fe2+/peroxydisulfate process. J Hazard Mater. 2012;215:138–45.
Norzaee S, Taghavi M, Djahed B, Kord Mostafapour F. Degradation of penicillin G by heat activated persulfate in aqueous solution. J Environ Manag. 2018;215:316–23.
Sizykh M, Batoeva A, Tsydenova O. UV-Activated Persulfate Oxidation of Orange III Dye Using KrCl Excilamp. CLEAN–Soil Air Water. 2018;46(4):1700187.
Wei C, Zhang J, Zhang Y, Zhang G, Zhou P, Li W, et al. Ultrasound enhanced heterogeneous activation of peroxymonosulfate by a co-NiOx catalyst. Water Sci Technol. 2017;76(6):1436–46.
Majidi S, Rahmani A. Determination of the efficiency of electro-Persulfate process in reducing concentration of ciprofloxacin from aquatic environment by Voltammetric measurement. World. 2018;7(1):19–24.
Ferkous H, Merouani S, Hamdaoui O, Pétrier C. Persulfate-enhanced sonochemical degradation of naphthol blue black in water: evidence of sulfate radical formation. Ultrason Sonochem. 2017;34:580–7.
Lin C-C, Lee L-T, Hsu L-J. Degradation of polyvinyl alcohol in aqueous solutions using UV-365 nm/S 2 O 8 2− process. Int J Environ Sci Technol. 2014;11(3):831–8.
Tarkwa J-B, et al. Photo-Fenton oxidation of Orange G azo dye: process optimization and mineralization mechanism. Environ Chem Lett. 2018;17:473–9.
Pulley S, van der Waal B, Rowntree K, Collins AL. Colour as reliable tracer to identify the sources of historically deposited flood bench sediment in the Transkei, South Africa: a comparison with mineral magnetic tracers before and after hydrogen peroxide pre-treatment. Catena. 2018;160:242–51.
Chatzitakis A, Berberidou C, Paspaltsis I, Kyriakou G, Sklaviadis T, Poulios I. Photocatalytic degradation and drug activity reduction of chloramphenicol. Water Res. 2008;42(1–2):386–94.
Elmolla ES, Chaudhuri M. Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2/TiO2 photocatalysis. Desalination. 2010;252(1–3):46–52.
Fan X, Hao H, Shen X, Chen F, Zhang J. Removal and degradation pathway study of sulfasalazine with Fenton-like reaction. J Hazard Mater. 2011;190(1–3):493–500.
Vaiopoulou E, Gikas P. Effects of chromium on activated sludge and on the performance of wastewater treatment plants: a review. Water Res. 2012;46(3):549–70.
Epold I, Trapido M, Dulova N. Degradation of levofloxacin in aqueous solutions by Fenton, ferrous ion-activated persulfate and combined Fenton/persulfate systems. Chem Eng J. 2015;279:452–62.
Farrokhi M, et al. Oxidation of pentachlorophenol by Fenton’s reagent. Iran J Public Health. 2003;32(1):6–10.
Yuan X, Guan R, Wu Z, Jiang L, Li Y, Chen X, et al. Effective treatment of oily scum via catalytic wet persulfate oxidation process activated by Fe 2+. J Environ Manag. 2018;217:411–5.
Qian Y, Xue G, Chen J, Luo J, Zhou X, Gao P, et al. Oxidation of cefalexin by thermally activated persulfate: kinetics, products, and antibacterial activity change. J Hazard Mater. 2018;354:153–60.
Roy D, et al. Composting leachate: characterization, treatment, and future perspectives. Rev Environ Sci Biotechnol. 2018:1–27.
Wu H, Feng Q, Yang H, Alam E, Gao B, Gu D. Modified biochar supported Ag/Fe nanoparticles used for removal of cephalexin in solution: characterization, kinetics and mechanisms. Colloids Surf A Physicochem Eng Asp. 2017;517:63–71.
Xu X-R, Li X-Z. Degradation of azo dye Orange G in aqueous solutions by persulfate with ferrous ion. Sep Purif Technol. 2010;72(1):105–11.
Fadaei A, Sadeghi M. Efficacy study on advanced oxidation processes (AOPs) application for pesticides removal from water with emphasis on their cost aspects. J Shahrekord Univ Med Sci. 2013;15(5):80–9.
Bansal P, Verma A. Pilot-scale single-step reactor combining photocatalysis and photo-Fenton aiming at faster removal of cephalexin. J Clean Prod. 2018;195:540–51.
Acknowledgments
The authors gratefully acknowledge the Kermanshah University of Medical Sciences (GrantNumber: 96397) for its financial support. We would like to specially thank from “Social development and Health Promotion Research Center”, Kermanshah University of Medical Sciences, for facilitating and providing us with invaluable guidance in performing and sharing the use of the possibility of their research center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Almasi, A., Esmaeilpoor, R., Hoseini, H. et al. Photocatalytic degradation of cephalexin by UV activated persulfate and Fenton in synthetic wastewater: optimization, kinetic study, reaction pathway and intermediate products. J Environ Health Sci Engineer 18, 1359–1373 (2020). https://doi.org/10.1007/s40201-020-00553-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40201-020-00553-1