Abstract
Summary
In this report, we present three cases of individuals from the same family with a diagnosis of CMT with severe tibia bone microarchitecture deterioration assessed by HR-pQCT. Charcot-Marie-Tooth disease (CMT) or hereditary neuropathy involves both motor and sensory nerves. Falls are often the first manifestation in these patients and represent an important risk factor for fracture. The reduction of mechanical input on bone inhibits bone formation by osteoblasts and accelerates bone resorption by osteoclasts, leading to disuse osteoporosis. We report three cases of individuals from the same family with a diagnosis of CMT with severe tibia bone microarchitecture deterioration assessed by high-resolution peripheral quantitative computed tomography (HR-pQCT). This affectation was exclusive to the tibia; the radius remained undamaged, showing the consequences of the lack of mobility and mechanical stimulation. Physical activity and rehabilitation, in addition to adequate calcium and vitamin D supplementation, may play an essential role in the management of this disease.
Similar content being viewed by others
Introduction
Charcot-Marie-Tooth disease (CMT), or hereditary motor and sensory neuropathy, is a genetically heterogeneous clinical polyneuropathy characterized by abnormal development of the peripheral nervous system [1,2,3]. Its pathophysiology includes different types of genetic mutations that affect proteins essential for nerve structure and/or function. According to the types of genetic mutations involved, the literature reports different patterns of inheritance and prevalence (8–41 per 100,000) [2, 4].
CMT patients may have an increased risk of fractures, associated with low bone mass and falls. Falls are often caused by muscle cramps, diminished deep tendon reflexes, impaired mobility, and the use of psychotropic drugs [5]. However, fracture risk is not well determined in these patients, and there are a few case reports in the literature [5, 6]. A retrospective cohort study showed that CMT patients had a 1.5-fold increased risk for fractures, mainly in hands, feet, and ankles [5].
In these patients, “bone disuse” is one of the factors leading to the development of osteoporosis. Physiologically, bones receive permanent mechanical stimuli from muscle. When there is an injury at that level, like in paraplegic patients, a rapid decrease in muscle mass is observed in response to adaptive changes, leading to disuse osteoporosis [7,8,9,10,11]. Patients with CMT might be affected by this mechanism because as their normal locomotion is impaired, muscle stimuli decrease. Moreover, being CMT an inherited disease, the onset occurs during childhood, and therefore, it probably interferes with the acquisition of final peak bone mass.
In this report, we discuss three members of the same family with early-onset CMT and alterations in bone microarchitecture assessed by high-resolution peripheral quantitative computed tomography (HR-pQCT) [12, 13]. Patients’ results were compared with a healthy female reference population [14]. Molecular detection of the index case was heterozygous variant MFN2 gene, c.280C> G (p.Arg94Gly), associated with autosomal dominant and recessive CMT neuropathy.
Case 1
A 39-year-old female patient was diagnosed with CMT during childhood, confined to a wheelchair since she was 34. She reported regular menstrual cycles while taking oral contraceptives. In the year 2013, she suffered a patella fracture as a consequence of a fall from her own height. In the interview, she did not report any other significant risk factors.
The patient had been previously evaluated in another institution where she was prescribed denosumab (subcutaneous 60 mg every 6 months for 4 years), calcium citrate (1500 mg/day), and vitamin D3 (400 UI/day). She consumed 500 mg/day of calcium in her diet. She was receiving kinesiotherapy and physiotherapy—though not regularly—to improve the functionality of the leg muscle groups. However, no positive changes were observed in areal bone mineral density (aBMD), assessed by dual-energy X-ray absorptiometry (DXA). For this reason, she was referred to our bone clinic. Biochemical and DXA results are shown in Tables 1 and 2.
Case 2
A 16-year-old female with CMT diagnosed in the setting of recurrent falls at age 12, 1 year before her menarche. Except for frequent falls, she had normal mobility. Her mother and aunt had the same diagnosis. She had a history of regular menses and no fractures. She consumed 500 mg/day of calcium in her diet and did not exercise on a regular basis. Biochemical results were within normal limits, except for CrossLaps (CTX) which were slightly above range and 25 OH-vitamin D below 30 ng/mL (Table 1). DXA results showed a BMD lower than expected for age in the lumbar spine and hip (Table 2).
Case 3
A 44-year-old female was diagnosed with CMT disease during childhood due to frequent falls. She had been confined to a wheelchair for the previous 4 years. She did not report any previous fractures or any other risk factors, had regular menses, and did not exercise or received physiotherapy on a regular basis. She consumed calcium citrate, (1500 mg/day), vitamin D3 (400 Ul/day), and 500 mg/day of calcium in her diet. In the previous 5 years, she had received zoledronic acid annually (5 mg intravenously). Biochemical results—which were within range—are shown in Table 1. BMD by DXA was lower than expected for age in the total hip (Table 2).
Bone microarchitecture measured by HR-pQCT
Volumetric bone mineral density and bone microarchitecture were assessed by high-resolution peripheral computed tomography (HR-pQCT) of the radius and distal tibia (Xtreme CT; Scanco Medical AG, Bassersdorf, Switzerland).
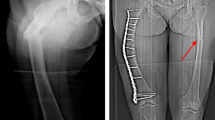
In the three patients, we found a significant asymmetry between the two regions evaluated. The distal radius showed normal trabecular and cortical values. At the tibia, we observed a severe deterioration of the trabecular compartment, with a significant fall in bone trabecular density and volume, as a consequence of decreased trabecular number and thickness with the subsequent increase in trabecular separation and bone heterogeneity (Fig. 1). These parameters were more than 50% lower than those of the reference population (Table 3). The cortical compartment showed a significant decrease in density and thickness in patient 1 and just a decrease in thickness in patient 3; patient 2 did not present any cortical alterations.
Discussion
We report three members of the same family with CMT disease with severe alteration of bone microarchitecture at the tibia, measured by HR-pQCT. CMT is a hereditary neuropathy that affects both motor and sensory nerves, leading to distal muscle weakness and atrophy, gait abnormalities and falls, and increased risk of fracture [1, 2, 15, 16]. HR-pQCT is a novel tool that, due to its high resolution, enables the assessment of trabecular and cortical compartments separately and measures bone microarchitecture parameters [13, 17, 18]. Our patients showed an asymmetry in bone microarchitecture involvement between the radius and the tibia, the latter being severely deteriorated. Consistently, the compromise of aBMD was more severe at the hip than at the lumbar spine, as has been previously described [9]. We have previously reported a case of disuse osteoporosis, with similar characteristics [19].
We found a severe deterioration of bone microarchitecture in our CMT patients, especially in the trabecular compartment but also in cortical bone. This affectation was exclusive to the tibia; the radius remained undamaged, showing the consequences of the lack of mobility and mechanical stimulation. These alterations were to be expected, considering the pathophysiology of this neuromuscular disease. Muscle provides an essential mechanical environment for the function and distribution of mineralized bone tissue [20,21,22,23,24]. Osteocytes, the most abundant bone cells, are positioned within the bone matrix in order to sense mechanical strain and translate it into chemical signals mediators to activate osteoblasts and osteoclasts [25,26,27,28]. This functional organization, capable of responding directionally to mechanical loads, was called mechanostat by Harold Frost [29]. In this mechanism, there is continuing feedback between muscle and bone tissues, with the osteocytes being the “sensors” that translate the stress and strains provoked by higher loads in increased bone formation. Then, those bones that bear higher weight are the ones that need higher bone mineral density, and this is achieved through the mechanostat mechanism [30].
Disuse osteoporosis was first described in 1973, to refer to a loss of bone mineral density as a consequence of lack of mechanical strain stimulus [24,25,26,27]. Although there is a wide variety of treatment options for osteoporosis—as bisphosphonates or denosumab—there is no evidence that they are effective to treat this particular kind of osteoporosis. Anabolic agents such as romosozumab or recombinant PTH might be more effective than antiresorptive therapies in patients with disuse osteoporosis [31,32,33]. Due to its different physiopathogeny, its treatment should focus on increasing the mechanical stimulus to activate the mechanostat mechanism [13,14,15, 33]. Rehabilitation, including therapeutic exercises and electrical stimulation, might be appropriate [20,21,22, 34]. In our patients treated with antiresorptive (cases 1 and 3), no changes were observed in BMD during follow-up. However, physical therapy had not been included as a part of the treatment. Physical activity and rehabilitation, in addition to adequate calcium and vitamin D supplementation, may play an essential role in the management of this disease.
In conclusion, we report three individuals with a diagnosis of CMT with severe tibia bone microarchitecture deterioration assessed by high-resolution peripheral quantitative computed tomography (HR-pQCT). This affectation was exclusive to the tibia; the radius remained undamaged, showing the consequences of the lack of mobility and mechanical stimulation.
References
Szigeti K, Lupski JR (2009) Charcot-Marie-Tooth disease. Eur J Hum Genet 17(6):703–710
Hoyle JC, Isfort MC, Roggenbuck J, Arnold WD (2015) The genetics of Charcot-Marie-Tooth disease: current trends and future implications for diagnosis and management. Appl Clin Genet 8:235–243
Louwerens JWK (2018) Operative treatment algorithm for foot deformities in Charcot-Marie-Tooth disease. Algorithmus für die operative Behandlung von Fußdeformitäten bei der Charcot-Marie-Tooth-Krankheit. Oper Orthop Traumatol 30(2):130–146
Timmerman V, Strickland AV, Züchner S (2014) Genetics of Charcot-Marie-Tooth (CMT) disease within the frame of the human genome project success. Genes (Basel) 5(1):13–32
Pouwels S, de Boer A, Leufkens HG, Weber WE, Cooper C, de Vries F (2014) Risk of fracture in patients with Charcot-Marie-Tooth disease. Muscle Nerve 50(6):919–924
Hsu JD (1979) Extremity fractures in children with neuromuscular disease. Johns Hopkins Med J 145(3):89–93
Aujla RS, Gulihar A, Taylor GJ (2008) Acromial stress fracture in a young wheelchair user with Charcot-Marie-Tooth disease: a case report. Cases J 1(1):359
Quintart C, Baillon JM, Libotte M (1999) Fracture pathologique du tibia compliquant une maladie de Charcot-Marie-Tooth [Pathologic fracture of the tibia associated with Charcot-Marie-Tooth disease]. Acta Orthop Belg 65(1):105–108
Garland DE, Stewart CA, Adkins RH, Hu SS, Rosen C, Liotta FJ, Weinstein DA (1992) Osteoporosis after spinal cord injury. J Orthop Res 10(3):371–378
Giannotti S, Bottai V, Dell’osso G et al (2013) Disuse osteoporosis of the upper limb: assessment of thirty patients. Clin Cases Miner Bone Metab 10(2):129–132
Robling AG, Castillo AB, Turner CH (2006) Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng 8(1):455–449
MacNeil JA, Boyd SK (2008) Improved reproducibility of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med Eng Phys 30:792–799
Nishiyama KK, Shane E (2013) Clinical imaging of bone microarchitecture with HR-pQCT. Curr Osteoporos Rep 11(2):147–155
Boutroy S, Bouxsein ML, Munoz F, Delmas PD (2005) In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 90(12):6508–6515
Guðmundsson B, Ólafsson E, Jakobsson F, Lúðvígsson P (2010) Prevalence of symptomatic Charcot-Marie-Tooth disease in Iceland: a study of a well-defined population. Neuroepidemiology 34:13–17
Palau F, Cuesta A, Pedrola L (2002) Avances en la genética molecular de las neuropatías hereditarias. Rev Neurol 35:246–253
Boutroy S, Van Rietbergen B, Sornay-Rendu E et al (2008) Finite element analysis based on in vivo hr-pqct images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res 23:392–399
Burghardt AJ, Link TM, Majumdar S (2011) High-resolution computed tomography for clinical imaging of bone microarchitecture. Clin Orthop Relat Res 469:2179–2193
Balonga MC, Zanchetta MB (2013) Grave deterioro de la microarquitectura ósea inducido por desuso y denervacion crónica. Actual Osteol 9(2):205–206
Keyak JH, Koyama AK, LeBlanc A, Lu Y, Lang TF (2009) Reduction in proximal femoral strength due to long-duration spaceflight. Bone. 44(3):449–453
Kannus P, Järvinen M, Sievänen H, Oja P, Vuori I (1994) Osteoporosis in men with a history of tibial fracture. J Bone Miner Res 9(3):423–429
Kazakia GJ, Tjong W, Nirody JA, Burghardt AJ, Carballido-Gamio J, Patsch JM, Link T, Feeley BT, Benjamin Ma C (2014) The influence of disuse on bone microstructure and mechanics assessed by HR-pQCT. Bone. 63:132–140
Alexandre C, Vico L (2011) Pathophysiology of bone loss in disuse osteoporosis. Joint Bone Spine 78(6):572–576
Takata S, Yasui N (2001) Disuse Osteoporosis. J Med Investig 48(3–4):147–156
Bellido T (2014) Osteocyte-driven bone remodeling. Calcif Tissue Int 94(1):25–34
Young MJ, Marshall A, Adams JE, Selby PL, Boulton AJ (1995) Osteopenia, neurological dysfunction, and the development of Charcot neuroarthropathy. Diabetes Care 18(1):34–38
Zhang K, Barragan-Adjemian C, Ye L, Kotha S, Dallas M, Lu Y, Zhao S, Harris M, Harris SE, Feng JQ, Bonewald LF (2006) E11/gp38 selective expression in osteocytes: regulation by mechanical strain and role in dendrite elongation. Mol Cell Biol 26(12):4539–4552
Winkler DG, Sutherland MK, Geoghegan JC et al (2003) Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22(23):6267–6276
Frost HM (1997) Defining osteopenias and osteoporoses: another view (with insights from a new paradigm). Bone. 20(5):385–391
Ferretti JL, Cointry GR, Capozza RF, Frost HM (2003) Bone mass, bone strength, muscle-bone interactions, osteopenias and osteoporoses. Mech Ageing Dev 124(3):269–279
Li CY, Price C, Delisser K, Nasser P, Laudier D, Clement M, Jepsen KJ, Schaffler MB (2005) Long-term disuse osteoporosis seems less sensitive to bisphosphonate treatment than other osteoporosis. J Bone Miner Res 20(1):117–124
Milsom S, Lin S-Y(S), Cornish J, Sharma S (2016) Disuse osteoporosis: a better understanding of pathophysiology may lead to potential therapies. J Diabetol Endocrinol 1(1):1–4
Kutilek S (2017) Denosumab treatment of severe disuse osteoporosis in a boy with spinal muscular atrophy. Acta Med Iran 55(10):658–660
Daly RM, Dalla Via J, Duckham RL, Fraser SF, Helge EW (2019) Exercise for the prevention of osteoporosis in postmenopausal women: an evidence-based guide to the optimal prescription. Braz J Phys Ther 23(2):170–180
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 24 kb)
Rights and permissions
About this article
Cite this article
Abdala, R., Levi, L., Longobardi, V. et al. Severe bone microarchitecture deterioration in a family with hereditary neuropathy: evidence of the key role of the mechanostat. Osteoporos Int 31, 2477–2480 (2020). https://doi.org/10.1007/s00198-020-05674-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-020-05674-9