Abstract
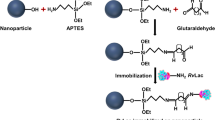
Halloysite nanotube (HNT) is a natural bio-compatible and stable nanomaterial available in abundance at low-cost. In this work, HNT was modified by two strategies to make it suitable for supporting immobilization of chloroperoxidase (CPO). Firstly, Fe3O4 nanoparticles were deposited on HNT, so magnetic separation can be used instead of centrifugation. Then, the magnetic HNT was modified by 3-aminopropyltriethoxysilane (APTES), which can provide amine group on surface of HNT and meanwhile inhibit the agglomeration of magnetic HNT. Then, HNT-Fe3O4 -APTES was linked with branched polyethyleneimine (PEI) to provide more amino for binding with enzyme. The so-prepared CPO@HNT-Fe3O4-APTES-PEI showed enhanced enzyme loading, reusability, improved thermal stability and tolerance to organic solvents than free CPO. For example, after 10 repeated uses, CPO@HNT- Fe3O4-APTES-PEI can maintain 92.20% of its original activity compared with 65.12% of activity of CPO@HNT-APTES-PEI and 45.69% of activity of CPO@HNT. The kinetic parameters indicated the affinity and specificity of immobilized enzyme to substrate was increased. CPO@HNT-Fe3O4-APTES-PEI was very efficient when it was applied in the degradation of pesticides mesotrione in wastewater. The degradation efficiency can reach 90% within 20 min at range of 5–40 μmol·L−1. These results ensure the potential practical application of this bio-materials in wastewater treatment.
Graphic Abstract











Similar content being viewed by others
References
Podsiadlo P, Kaushik AK, Arruda EM, Waas AM, Shim BS, Xu J et al (2007) Ultrastrong and stiff layered polymer nanocomposites. Science 318:80–83. https://doi.org/10.1126/science.1143176
Li YC, Schulz J, Mannen S, Delhom C, Condon B, Chang S et al (2010) Flame retardant behavior of polyelectrolyte-clay thin film assemblies on cotton fabric. ACS Nano 4:3325–3337. https://doi.org/10.1021/nn100467e
Ruiz-Hitzky E, Darder M, Fernandes FM, Wicklein B, Alcântara ACS, Aranda P (2013) Fibrous clays based bionanocomposites. Prog Polym Sci 38:1392–1414. https://doi.org/10.1016/j.progpolymsci.2013.05.004
Lvov Y, Wang W, Zhang L, Fakhrullin R (2016) Halloysite clay nanotubes for loading and sustained release of functional compounds. Adv Mater 28:1227–1250. https://doi.org/10.1002/adma.201502341
Rouster P, Dondelinger M, Galleni M, Nysten B, Jonas AM, Glinel K (2019) Layer-by-layer assembly of enzyme-loaded halloysite nanotubes for the fabrication of highly active coatings. Colloid Surf B 178:508–514. https://doi.org/10.1016/j.colsurfb.2019.03.046
Zhai R, Zhang B, Liu L, Xie Y, Zhang H, Liu J (2010) Immobilization of enzyme biocatalyst on natural halloysite nanotubes. Catal Commun 12:259–263. https://doi.org/10.1016/j.catcom.2010.09.030
Zhai R, Zhang B, Wan Y, Li C, Wang J, Liu J (2013) Chitosan-halloysite hybrid-nanotubes: horseradish peroxidase immobilization and applications in phenol removal. Chem Eng J 214:304–309. https://doi.org/10.1016/j.cej.2012.10.073
Chao C, Liu J, Wang J, Zhang Y, Zhang B, Zhang Y et al (2013) Surface modification of halloysite nanotubes with dopamine for enzyme immobilization. ACS Appl Mater Int 5:10559–10564. https://doi.org/10.1021/am4022973
Fan X, Hu M, Li S, Zhai Q, Wang F, Jiang Y (2018) Charge controlled immobilization of chloroperoxidase on both inner/outer wall of NHT: improved stability and catalytic performance in the degradation of pesticide. Appl Clay Sci 163:92–99. https://doi.org/10.1016/j.clay.2018.07.016
Morris DR, Hager LP (1966) Chloroperoxidase, I. Isolation and properties of the crystalline glycoprotein. J Biol Chem 241:1763–1768
Gopalakrishnan K, Roostaei J, Zhang Y (2018) Mixed culture of Chlorella sp. and wastewater wild algae for enhanced biomass and lipid accumulation in artificial wastewater medium. Front Environ Sci Eng 12(4):14. https://doi.org/10.1007/s11783-018-1075-2
Gao F, Jiang Y, Hu M, Li S, Zhai Q (2016) Bienzymatic nanoreactors composed of chloroperoxidase–glucose oxidase on Au@Fe3O4 nanoparticles: dependence of catalytic performance on the bioarchitecture. Mater Des 111:414–420. https://doi.org/10.1016/j.matdes.2016.09.02
Hormozi Jangi SR, Akhond M, Dehghani Z (2020) High throughput covalent immobilization process for improvement of shelf-life, operational cycles, relative activity in organic media and enzymatic kinetics of urease and its application for urea removal from water samples. Process Biochem 90:102–112. https://doi.org/10.1016/j.procbio.2019.11.001
Suo H, Xu L, Xue Y, Qiu X, Huang H, Hu Y (2020) Ionic liquids-modified cellulose coated magnetic nanoparticles for enzyme immobilization: improvement of catalytic performance. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2020.115914
Liu DM, Dong C (2020) Recent advances in nano-carrier immobilized enzymes and their applications. Process Biochem 92:464–475. https://doi.org/10.1016/j.procbio.2020.02.005
Mishra A, Melo JS, Agrawal A, Kashyap Y, Sen D (2020) Preparation and application of silica nanoparticles-Ocimum basilicum seeds bio-hybrid for the efficient immobilization of invertase enzyme. Colloids Surf B 188:110796. https://doi.org/10.1016/j.colsurfb.2020.110796
Flores EEE, Cardoso FD, Siqueira LB, Ricardi NC, Costa TH, Rodrigues RC, Klein MP, Hertz PF (2019) Influence of reaction parameters in the polymerization between genipin and chitosan for enzyme immobilization. Process Biochem 84:73–80. https://doi.org/10.1016/j.procbio.2019.06.001
Binhayeeding N, Yunu T, Pichid N, Klomklao S, Sangkharak K (2020) Immobilisation of Candida rugosa lipase on polyhydroxybutyrate via a combination of adsorption and cross-linking agents to enhance acylglycerol production. Process Biochem. https://doi.org/10.1016/j.procbio.2020.02.007
Zhou L, Luo X, Li J, Ma L, He Y, Jiang Y, Yin L, Gao L (2019) Meso-molding three-dimensionally ordered macroporous alumina: a new platform to immobilize enzymes with high performance. Biochem Eng J 146:60–68. https://doi.org/10.1016/j.bej.2019.03.002
Mahmoodi NM, Saffar-Dastgerdi MH, Hayati B (2020) Environmentally friendly novel covalently immobilized enzyme bionanocomposite: from synthesis to the destruction of pollutant. Compos B Eng 184:107666. https://doi.org/10.1016/j.compositesb.2019.107666
Sanjana S, Medha MU, Meghna MR, Shruthi TS, Srinivas SP, Madhyastha H et al (2019) Enzyme immobilization on quercetin capped gold and silver nanoparticles for improved performance. Mater Today Proc 10:92–99. https://doi.org/10.1016/j.matpr.2019.02.193
Wang L, Guan S, Bai J, Jiang Y, Song Y, Zheng X, Gao J (2020) Enzyme immobilized in BioMOFs: facile synthesis and improved catalytic performance. Int J Biol Macromol 144:19–28. https://doi.org/10.1016/j.ijbiomac.2019.12.054
Dal Magro L, Kornecki JF, Klein MP, Rodrigues RC (2020) Pectin lyase immobilization using the glutaraldehyde chemistry increases the enzyme operation range. Enzyme Microb Technol 132:109397. https://doi.org/10.1016/j.enzmictec.2019.109397
Wang D, Jiang W (2019) Preparation of chitosan-based nanoparticles for enzyme immobilization. Int J Biol Macromol 126:1125–1132. https://doi.org/10.1016/j.ijbiomac.2018.12.243
Monajati M, Borandeh S, Hesami A, Mansouri D, Tamaddon AM (2018) Immobilization of L-asparaginase on aspartic acid functionalized graphene oxide nanosheet: enzyme kinetics and stability studies. Chem Eng J 354:1153–1163. https://doi.org/10.1016/j.cej.2018.08.058
Poorakbar E, Shafiee A, Saboury AA, Rad BL, Khoshnevisan K, Ma’mani L, Derakhshankhah H, Ganjali MR, Hosseini M (2018) Synthesis of magnetic gold mesoporous silica nanoparticles core shell for cellulase enzyme immobilization: improvement of enzymatic activity and thermal stability. Process Biochem 71:92–100. https://doi.org/10.1016/j.procbio.2018.05.012
Mei S, Han P, Wu H, Shi J, Tang L, Jiang Z (2018) One-pot fabrication of chitin-shellac composite microspheres for efficient enzyme immobilization. J Biotechnol 266:1–8. https://doi.org/10.1016/j.jbiotec.2017.11.015
Ulu A, Ozcan I, Koytepe S, Ates B (2018) Design of epoxy-functionalized Fe3O4@MCM-41 core–shell nanoparticles for enzyme immobilization. Int J Biol Macromol 115:1122–1130. https://doi.org/10.1016/j.ijbiomac.2018.04.157
Zhu X, He B, Zhao C, Ma Y, Yang W (2018) Simultaneously and separately immobilizing incompatible dual-enzymes on polymer substrate via visible light induced graft polymerization. Appl Surf Sci 436:73–79. https://doi.org/10.1016/j.apsusc.2017.11.284
Chen Z, Wang X, Chen Y, Xue Z, Guo Q, Ma Q, Chen H (2018) Preparation and characterization of a novel nanocomposite with double enzymes immobilized on magnetic Fe3O4 -chitosan-sodium tripolyphosphate. Colloids Surf B 169:280–288. https://doi.org/10.1016/j.colsurfb.2018.04.066
Yao J, Wang Q, Wang Y, Zhang Y, Zhang B, Zhang H (2015) Immobilization of laccase on chitosan–halloysite hybrid porous microspheres for phenols removal. Desalin Water Treat 55:1293–1301. https://doi.org/10.1080/19443994.2014.923337
Acknowledgments
This work is supported by the National Natural Science Foundation of China (21873061).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, X., Fan, X., Wang, Y. et al. Amino modified magnetic halloysite nanotube supporting chloroperoxidase immobilization: enhanced stability, reusability, and efficient degradation of pesticide residue in wastewater. Bioprocess Biosyst Eng 44, 483–493 (2021). https://doi.org/10.1007/s00449-020-02458-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02458-7