Abstract
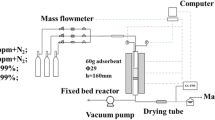
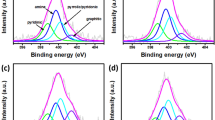
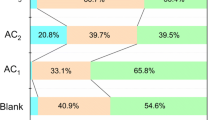
Elevated temperature pressure swing adsorption is a newly developed gas purification technology, wherein the product gas recovery rate can be significantly improved by adding a steam rinse and purge. Furthermore, high temperature industrial syngas can be directly purified at elevated temperatures, so the temperature variation during purification process is not as large as that in Selexol, Rectisol or some other methods, which benefits to the simplification of heat management and improvement of energy efficiency. At working temperatures of approximately 200 °C, activated carbon is considered to be a promising adsorbent. Nevertheless, activated carbon also adsorbs large amounts of steam during rinse and purge, which affects its adsorption capacity. Herein, hydrophobic activated carbon was synthesized by impregnation and characterized, achieving a contact angle with a water droplet of up to 134°. When exposed to wet air, the weight increase of the hydrophobic adsorbent was 0.3%, whereas it was approximately 12% for the commercial activated carbon, which can be mainly attributed to vapor adsorption. The CO2 adsorption capacity was tested under humid conditions at 200 °C on a fixed bed. The effect of steam on the hydrophobic adsorbent was much smaller than that on the commercial adsorbent. The working capacity decreased from 0.091 to 0.009 mmol/g when the commercial activated carbon was operated under steam at 1 atm, whereas the hydrophobic material exhibited a stable and desired working capacity at the same condition, which was around 0.05 mmol/g. Steam rinse and purge were demonstrated to be feasible for the hydrophobic adsorbent and the introducing of steam rinse could not only compensate for the deterioration of adsorption capacity resulting from impregnation, but further improve the purification efficiency.








Similar content being viewed by others
References
Alptekin, G.: A Low Cost, High Capacity Regenerable Sorbent for Pre-combustion CO2 Capture. TDA Research, Golden (2012)
Anwar, M., Hogarth, C.A., Bulpett, R.: An XPS study of amorphous MoO3/SiO films deposited by co-evaporation. J. Mater. Sci. 25, 1784–1788 (1990)
Boon, J., Cobden, P.D., Van Dijk, H.A.J., Hoogland, C., van Selow, E.V., van Sint Annaland, M.: Isotherm model for high-temperature, high-pressure adsorption of CO2 and H2O on K-promoted hydrotalcite. Chem. Eng. J. 248, 406–414 (2014)
Boon, J., Cobden, P.D., Van Dijk, H.A.J., van Sint Annaland, M.: High-temperature pressure swing adsorption cycle design for sorption-enhanced water–gas shift. Chem. Eng. Sci. 122, 219–231 (2015)
Chaubey, R., Sahu, S., James, O.O., Maity, S.: A review on development of industrial processes and emerging techniques for production of hydrogen from renewable and sustainable sources. Renew. Sustain. Energy Rev. 23, 443–462 (2013)
Coenen, K., Gallucci, F., Pio, G., Cobden, P., van Dijk, E., Hensen, E., van Sint Annaland, M.: On the influence of steam on the CO2 chemisorption capacity of a hydrotalcite-based adsorbent for SEWGS applications. Chem. Eng. J. 314, 554–569 (2017)
Do, D.D., Do, H.D.: A model for water adsorption in activated carbon. Carbon 38, 767–773 (2000)
Fletcher, A.J., Yüzak, Y., Thomas, K.M.: Adsorption and desorption kinetics for hydrophilic and hydrophobic vapors on activated carbon. Carbon 44, 989–1004 (2006)
Fu, Q., Yan, H., Shen, Y., Qin, Y., Zhang, D., Zhou, Y.: Optimal design and control of pressure swing adsorption process for N2/CH4 separation. J. Clean. Prod. 170, 704–714 (2018)
Hagio, T., Takase, A., Umebayashi, S.: X-ray photoelectron spectroscopic studies of β-sialons. J. Mater. Sci. Lett. 11, 878–880 (1992)
Hao, P., Shi, Y., Li, S., Zhu, X., Cai, N.: Correlations between adsorbent characteristics and the performance of pressure swing adsorption separation process. Fuel 230, 9–17 (2018)
Horikawa, T., Sekida, T., Hayashi, J.I., Katoh, M., Do, D.D.: A new adsorption–desorption model for water adsorption in porous carbons. Carbon 49, 416–424 (2011)
Horikawa, T., Sakao, N., Sekida, T., Hayashi, J.I., Do, D.D., Katoh, M.: Preparation of nitrogen-doped porous carbon by ammonia gas treatment and the effects of N-doping on water adsorption. Carbon 50, 1833–1842 (2012)
Khunpolgrang, J., Yosantea, S., Kongnoo, A., Phalakornkule, C.: Alternative PSA process cycle with combined vacuum regeneration and nitrogen purging for CH4/CO2 separation. Fuel 140, 171–177 (2015)
Li, S., Hao, P., Zhu, X., Shi, Y., Cai, N., Li, S., Jiang, H.: On-site demonstration of an elevated temperature hydrogen clean-up unit for fuel cell applications. Adsorption 25, 1683–1693 (2019)
Ling, J., Ntiamoah, A., Xiao, P., Webley, P.A., Zhai, Y.: Effects of feed gas concentration, temperature and process parameters on vacuum swing adsorption performance for CO2 capture. Chem. Eng. J. 265, 47–57 (2015)
Liu, H.B., Yang, B., Xue, N.D.: Enhanced adsorption of benzene vapor on granular activated carbon under humid conditions due to shifts in hydrophobicity and total micropore volume. J. Hazard. Mater. 318, 425–432 (2016)
Liu, L., Tan, S.J., Horikawa, T., Do, D.D., Nicholson, D., Liu, J.: Water adsorption on carbon—a review. Adv. Colloid Interface Sci. 250, 64–78 (2017)
Luberti, M., Friedrich, D., Brandani, S., Ahn, H.: Design of a H2 PSA for cogeneration of ultrapure hydrogen and power at an advanced integrated gasification combined cycle with pre-combustion capture. Adsorption 20, 511–524 (2014)
Nikolaidis, G.N., Kikkinides, E.S., Georgiadis, M.C.: Model-based approach for the evaluation of materials and processes for post-combustion carbon dioxide capture from flue gas by PSA/VSA processes. Ind. Eng. Chem. Res. 55, 635–646 (2016)
Reijers, R., van Selow, E., Cobden, P., Boon, J., van den Brink, R.: SEWGS process cycle optimization. Energy Procedia 4, 1155–1161 (2011)
Rutherford, S.W.: Modeling water adsorption in carbon micropores: study of water in carbon molecular sieves. Langmuir 22, 702–708 (2006)
Sircar, S., Golden, T.C.: Purification of hydrogen by pressure swing adsorption. Sep. Sci. Technol. 35, 667–687 (2000)
Sullivan, P.D., Stone, B.R., Hashisho, Z., Rood, M.J.: Water adsorption with hysteresis effect onto microporous activated carbon fabrics. Adsorption 13, 173–189 (2007)
Voldsund, M., Jordal, K., Anantharaman, R.: Hydrogen production with CO2 capture. Int. J. Hydrogen Energy 41, 4969–4992 (2016)
Yin, Q., Jay, K., Lin, Y.S.: Oxygen sorption and desorption properties of Sr–Co–Fe oxide. Chem. Eng. Sci. 63, 2211–2218 (2008)
Yu, X.R., Hantsche, H.: Vertical differential charging in monochromatized small spot X-ray photoelectron spectroscopy. Surf. Interface Anal. 20, 555–558 (1993)
Yu, C.H., Huang, C.H., Tan, C.S.: A review of CO2 capture by absorption and adsorption. Aerosol Air Qual. Res. 12, 745–769 (2012)
Yuan, B., Wu, X., Chen, Y., Huang, J., Luo, H., Deng, S.: Adsorption of CO2, CH4, and N2 on ordered mesoporous carbon: approach for greenhouse gases capture and biogas upgrading. Environ. Sci. Technol. 47, 5474–5480 (2013)
Zhao, Y., Yao, K.X., Teng, B., Zhang, T., Han, Y.: A perfluorinated covalent triazine-based framework for highly selective and water–tolerant CO2 capture. Energy Environ. Sci. 6, 3684–3692 (2013)
Zapata, P.A., Faria, J., Ruiz, M.P., Jentoft, R.E., Resasco, D.E.: Hydrophobic zeolites for biofuel upgrading reactions at the liquid–liquid interface in water/oil emulsions. J. Am. Chem. Soc. 134, 8570–8578 (2012)
Zhu, X., Shi, Y., Cai, N.: Integrated gasification combined cycle with carbon dioxide capture by elevated temperature pressure swing adsorption. Appl. Energy 176, 196–208 (2016)
Zhu, X., Shi, Y., Li, S., Cai, N.: Two-train elevated-temperature pressure swing adsorption for high-purity hydrogen production. Appl. Energy 229, 1061–1071 (2018)
Zhu, X., Li, S., Shi, Y., Cai, N.: Recent advances in elevated-temperature pressure swing adsorption for carbon capture and hydrogen production. Prog. Energy Combust. Sci. 75, 100784 (2019)
Acknowledgements
This research was financed by National Key Research Development Program of China (No. 2018YFC0810001), the National Natural Science Foundation of China (No. 51806120), the Seed Fund of Shanxi Research Institute for Clean Energy, Tsinghua University and Shanxi Province Science and Technology Major Projects (MH2015-06).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hao, P., Shi, Y., Li, S. et al. Hydrophobic activated carbon for elevated-temperature pressure swing adsorption. Adsorption 26, 1093–1100 (2020). https://doi.org/10.1007/s10450-020-00223-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-020-00223-6