The Neutrophil-to-Monocyte Ratio and Lymphocyte-to-Neutrophil Ratio at Admission Predict In-Hospital Mortality in Mexican Patients with Severe SARS-CoV-2 Infection (Covid-19)
Abstract
:1. Introduction
2. Material and Methods
2.1. Subjects
2.2. Data Collection
2.3. Laboratory Measures
2.4. Statistics
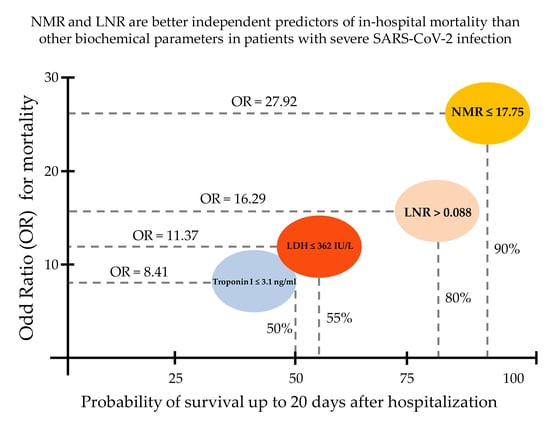
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lai, C.-C.; Shih, T.-P.; Ko, W.-C.; Tang, H.-J.; Hsueh, P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef] [PubMed]
- Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi 2020, 41, 145–151. [Google Scholar]
- Yan, Y.; Shin, W.I.; Pang, Y.X.; Meng, Y.; Lai, J.; You, C.; Zhao, H.; Lester, E.; Wu, T.; Pang, C.H. The First 75 Days of Novel Coronavirus (SARS-CoV-2) Outbreak: Recent Advances, Prevention, and Treatment. Int. J. Environ. Res. Public Health 2020, 17, 2323. [Google Scholar] [CrossRef] [Green Version]
- Ceylan, Z. Estimation of COVID-19 prevalence in Italy, Spain, and France. Sci. Total. Environ. 2020, 729, 138817. [Google Scholar] [CrossRef] [PubMed]
- Vandoros, S. Excess mortality during the Covid-19 pandemic: Early evidence from England and Wales. Soc. Sci. Med. 2020, 258, 113101. [Google Scholar] [CrossRef]
- Suárez, V.; Quezada, M.S.; Ruiz, S.O.; De Jesús, E.R. Epidemiology of COVID-19 in Mexico: From the 27th of February to the 30th of April 2020. Rev. Clínica Española Engl. Ed. 2020. [Google Scholar] [CrossRef]
- Friedman, J.; Calderón-Villarreal, A.; Bojorquez, I.; Hernández, C.V.; Schriger, D.L.; Hirashima, E.T. Excess Out-Of-Hospital Mortality and Declining Oxygen Saturation: The Sentinel Role of EMS Data in the COVID-19 Crisis in Tijuana, Mexico. Ann. Emerg. Med. 2020. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, X.; Fan, Q.; Liu, H.; Liu, X.; Liu, Z.; Zhang, Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J. Thromb. Haemost. 2020, 18, 1324–1329. [Google Scholar] [CrossRef]
- Lin, Z.; Long, F.; Yang, Y.; Chen, X.; Xu, L.; Yang, M. Serum ferritin as an independent risk factor for severity in COVID-19 patients. J. Infect. 2020, 81, 647–679. [Google Scholar] [CrossRef]
- Sahu, B.R.; Kampa, R.K.; Padhi, A.; Panda, A.K. C-reactive protein: A promising biomarker for poor prognosis in COVID-19 infection. Clin. Chim. Acta 2020, 509, 91–94. [Google Scholar] [CrossRef]
- Paul, P. Cardiac Troponin-I may be a predictor of complications and mortality in COVID-19 patients. Curr. Med. Res. Pract. 2020, 10, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; Aggarwal, G.; Wong, J.; Benoit, S.; Vikse, J.; Plebani, M.; Lippi, G. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis. Am. J. Emerg. Med. 2020, 38, 1722–1726. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Han, C.; Pei, S.; Yin, M.; Chen, X. Procalcitonin levels in COVID-19 patients. Int. J. Antimicrob. Agents 2020, 56, 106051. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Li, R.; Wu, X.; Zhao, Y.; Wang, T.; Zheng, Z.; Zeng, S.; Ding, X.; Nie, H. Clinical features in 52 patients with COVID-19 who have increased leukocyte count: A retrospective analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 1–9. [Google Scholar] [CrossRef]
- Zhao, Y.; Nie, H.-X.; Hu, K.; Wu, X.-J.; Zhang, Y.-T.; Wang, M.-M.; Wang, T.; Zheng, Z.-S.; Li, X.-C.; Zeng, S.-L. Abnormal immunity of non-survivors with COVID-19: Predictors for mortality. Infect. Dis. Poverty 2020, 9, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.; Du, X.; Chen, J.; Jin, Y.; Peng, L.; Wang, H.H.; Luo, M.; Chen, L.; Zhao, Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020, 81, e6–e12. [Google Scholar] [CrossRef]
- Belice, T.; Demir, I.; Yüksel, A. Role of neutrophil-lymphocyte-ratio in the mortality of males diagnosed with COVID-19. Iran. J. Microbiol. 2020, 12, 194–197. [Google Scholar] [CrossRef]
- Grenader, T.; Pavel, M.E.; Ruszniewski, P.B.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; et al. Prognostic value of the neutrophil/lymphocyte ratio in enteropancreatic neuroendocrine tumors. Anti-Cancer Drugs 2020, 31, 216–222. [Google Scholar] [CrossRef]
- Ventriglia, J.; Petrillo, A.; Alváro, M.H.; Laterza, M.M.; Savastano, B.; Gambardella, V.; Tirino, G.; Pompella, L.; Diana, A.; Iovino, F.; et al. Neutrophil to Lymphocyte Ratio as a Predictor of Poor Prognosis in Metastatic Pancreatic Cancer Patients Treated with Nab-Paclitaxel plus Gemcitabine: A Propensity Score Analysis. Gastroenterol. Res. Pract. 2018, 2018, 2373868. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.; Kanetsky, P.A.; Egan, K.M. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer. Sci. Rep. 2019, 9, 19670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Li, S.; Liu, J.; Liang, B.; Wang, X.; Wang, H.; Li, W.; Tong, Q.; Yi, J.; Zhao, L.; et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020, 55, 102763. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, F.; Wang, X.; Yan, J.; Zhu, F.; Tang, S.; Deng, Y.; Wang, H.; Chen, R.; Yu, Z.; et al. Neutrophil to lymphocyte ratio as prognostic and predictive factor in patients with coronavirus disease 2019: A retrospective cross-sectional study. J. Med Virol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Basbus, L.; I Lapidus, M.; Martingano, I.; Puga, M.C.; Pollán, J. Neutrophil to lymphocyte ratio as a prognostic marker in COVID-19. Medicina (B Aires) 2020, 80 (Suppl. 3), 31–36. [Google Scholar] [PubMed]
- Tatum, D.; Taghavi, S.; Houghton, A.; Stover, J.; Toraih, E.; Duchesne, J. Neutrophil-to-Lymphocyte Ratio and Outcomes in Louisiana Covid-19 Patients. Shock 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-J.; Cao, Y.-Y.; Tan, G.; Dong, X.; Wang, B.-C.; Lin, J.; Yan, Y.-Q.; Liu, G.-H.; Akdis, M.; Akdis, C.A.; et al. Clinical, radiological and laboratory characteristics and risk factors for severity and mortality of 289 hospitalized COVID-19 patients. Allergy 2020. [Google Scholar] [CrossRef]
- Gormez, S.; Ekicibasi, E.; Degirmencioglu, A.; Paudel, A.; Erdim, R.; Gumusel, H.K.; Eroglu, E.; Tanboga, I.H.; Dagdelen, S.; Sariguzel, N.; et al. Association between renin–angiotensin–aldosterone system inhibitor treatment, neutrophil–lymphocyte ratio, D-Dimer and clinical severity of COVID-19 in hospitalized patients: A multicenter, observational study. J. Hum. Hypertens. 2020. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, X.; Kong, M.; Mao, X.; Huang, L.; He, P.; Pan, S.; Li, J.; Lu, Z. Clinical and hematological characteristics of 88 patients with COVID-19. Int. J. Lab. Hematol. 2020. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Hemmat, N.; Derakhshani, A.; Baghi, H.B.; Silvestris, N.; Baradaran, B.; De Summa, S. Neutrophils, Crucial, or Harmful Immune Cells Involved in Coronavirus Infection: A Bioinformatics Study. Front. Genet. 2020, 11, 641. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, S.; Sun, Q.; Zhu, J.; Chen, B.; Xiong, M.; Cao, G. Immune-Inflammatory Parameters in COVID-19 Cases: A Systematic Review and Meta-Analysis. Front. Med. 2020, 7, 301. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, A.; Verhagen, J.; Blaser, K.; Akdis, M.; Akdis, C.A. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: The role of T regulatory cells. Immunology 2006, 117, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.M.; Liu, Y.-J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef]
- Devêvre, E.F.; Renovato-Martins, M.; Clément, K.; Sautès-Fridman, C.; Cremer, I.; Poitou, C. Profiling of the Three Circulating Monocyte Subpopulations in Human Obesity. J. Immunol. 2015, 194, 3917–3923. [Google Scholar] [CrossRef]
- Mukherjee, R.; Barman, P.K.; Thatoi, P.K.; Tripathy, R.; Das, B.K.; Ravindran, B. Non-Classical monocytes display inflammatory features: Validation in Sepsis and Systemic Lupus Erythematous. Sci. Rep. 2015, 5, 13886. [Google Scholar] [CrossRef] [Green Version]
- Grün, J.L.; Manjarrez-Reyna, A.N.; Gómez-Arauz, A.Y.; León-Cabrera, S.; Rückert, F.; Fragoso, J.M.; Bueno-Hernández, N.; Islas-Andrade, S.; Melendez, G.; Escobedo, G. High-Density Lipoprotein Reduction Differentially Modulates to Classical and Nonclassical Monocyte Subpopulations in Metabolic Syndrome Patients and in LPS-Stimulated Primary Human Monocytes In Vitro. J. Immunol. Res. 2018, 2018, 2737040. [Google Scholar] [CrossRef]
- Gatti, A.; Radrizzani, D.; Viganò, P.; Mazzone, A.; Brando, B. Decrease of non-classical and intermediate monocyte subsets in severe acute SARS-CoV -2 infection. Cytom. Part A 2020, 97, 887–890. [Google Scholar] [CrossRef]
- Duque, G.A.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [Green Version]
- Sanyaolu, A.; Okorie, C.; Marinkovic, A.; Patidar, R.; Younis, K.; Desai, P.; Hosein, Z.; Padda, I.; Mangat, J.; Altaf, M. Comorbidity and its Impact on Patients with COVID-19. SN Compr. Clin. Med. 2020, 2, 1069–1076. [Google Scholar] [CrossRef] [PubMed]





| Parameters | Survivors (n = 34) | Non-Survivors (n = 20) | p Value |
|---|---|---|---|
| Gender (W/M) | 21/13 | 5/15 | 0.002 * |
| Age (years) | 54.06 ± 12.43 | 62.9 ± 14.18 | 0.020 * |
| BMI (kg/m2) | 28.24 ± 4.60 | 27.88 ± 4.05 | 0.903 |
| Obesity prevalence (%) | 52.17 | 41.66 | 0.277 |
| T2D prevalence (%) | 43.47 | 75.00 | 0.037 * |
| Hypertension prevalence (%) | 17.39 | 58.33 | 0.006 * |
| Coronary heart disease (%) | 8.69 | 33.33 | 0.033 * |
| IMV (%) | 30.43 | 83.33 | 0.001 * |
| Time to extubation (days) | 2.43 ± 0.79 | 3.66 ± 0.82 | 0.167 |
| Inpatient days (days) | 15.65 ± 3.13 | 8.41 ± 1.66 | 0.060 |
| Drug regimen | Aziythromycin, ceftriaxone, enoxaparin sodium, dexamethasone, and acetaminophen | - | |
| Parameters | Survivors (n = 34) | Non-Survivors (n = 20) | p Value |
|---|---|---|---|
| Glucose (mg/dL) | 148.19 ± 92.13 | 148.16 ± 60.76 | 0.999 |
| Urea (mg/dL) | 42.05 ± 37.06 | 91.07 ± 77.16 | 0.004 * |
| Creatinine (mg/dL) | 0.995 ± 1.18 | 1.85 ± 2.79 | 0.133 |
| Uric Acid (mg/dL) | 5.65 ± 2.81 | 7.39 ± 4.09 | 0.097 |
| Total Cholesterol (mg/dL) | 151.77 ± 34.98 | 126.06 ± 20.85 | 0.010 * |
| Triglycerides (mg/dL) | 166.04 ± 67.93 | 169.75 ± 54.89 | 0.853 |
| HDL (mg/dL) | 34.30 ± 10.88 | 23.92 ± 12.09 | 0.015 * |
| LDL (mg/dL) | 95.30 ± 30.14 | 70.38 ± 18.69 | 0.012 * |
| Total bilirubin (mg/dL) | 0.684 ± 0.401 | 0.804 ± 0.315 | 0.349 |
| Direct bilirubin (mg/dL) | 0.185 ± 0.133 | 0.323 ± 0.215 | 0.012 * |
| Indirect bilirubin (mg/dL) | 0.492 ± 0.254 | 0.498 ± 0.153 | 0.939 |
| ALT (IU/L) | 35.42 ± 26.29 | 41.47 ± 28.92 | 0.465 |
| AST (IU/L) | 34.74 ± 22.86 | 59 ± 47.74 | 0.021 * |
| ALP (IU/L) | 91.00 ± 27.22 | 125.29 ± 120.86 | 0.134 |
| GGT (IU/L) | 69.54 ± 43.07 | 124.33 ± 101.26 | 0.015 * |
| Total Protein (mg/dL) | 6.59 ± 0.525 | 6.31 ± 0.627 | 0.099 |
| Albumin (mg/dL) | 3.59 ± 0.479 | 2.94 ± 0.409 | 0.001 * |
| LDH (IU/L) | 320.63 ± 132.11 | 475.63 ± 195.83 | 0.001 * |
| Amylase (IU/L) | 46.1 ± 35.39 | 56.83 ± 26.93 | 0.429 |
| Lipase (IU/L) | 116.00 ± 304.00 | 52.36 ± 52.27 | 0.502 |
| CPK (IU/L) | 101.52 ± 97.49 | 699.57 ± 1937.82 | 0.114 |
| CK-MB (IU/L) | 23.80 ± 11.90 | 44.43 ± 59.39 | 0.099 |
| Phosphorus (mg/dL) | 3.89 ± 2.01 | 4.25 ± 1.67 | 0.516 |
| Magnesium (mg/dL) | 2.74 ± 3.53 | 2.42 ± 0.629 | 0.698 |
| Sodium (mEq/L) | 136.00 ± 6.56 | 138.53 ± 6.31 | 0.182 |
| Potassium (mEq/L) | 5.56 ± 6.67 | 4.47 ± 0.719 | 0.484 |
| Chlorine (mEq/L) | 100.28 ± 7.19 | 101.84 ± 6.49 | 0.441 |
| Calcium (mg/dL) | 8.63 ± 0.686 | 8.14 ± 1.20 | 0.076 |
| Parameters | Survivors (n = 34) | Non-Survivors (n = 20) | p Value |
|---|---|---|---|
| Prothrombine time (s) | 11.97 ± 2.49 | 12.96 ± 1.55 | 0.237 |
| INR | 0.995 ± 0.246 | 1.10 ± 0.167 | 0.216 |
| Thrombin time (s) | 16.76 ± 1.61 | 17.78 ± 1.42 | 0.085 |
| aPTT (s) | 26.18 ± 6.99 | 26.36 ± 5.68 | 0.941 |
| Fibrinogen (mg/dL) | 637.23 ± 222.29 | 703.75 ± 206.92 | 0.400 |
| D-dimer (Ug/L) | 975.33 ± 477.81 | 8260.33 ± 13354.39 | 0.017 * |
| Ferritin (ng/mL) | 522.62 ± 451.93 | 937.00 ± 415.09 | 0.012 * |
| CRP (mg/L) | 129.43 ± 90.10 | 221.07 ± 108.32 | 0.014 * |
| Troponin I (ng/mL) | 37.68 ± 63.57 | 68.458 ± 112.73 | 0.338 |
| Myoglobine (ng/L) | 94.49 ± 107.76 | 254.38 ± 353.69 | 0.089 |
| Procalcitonin (ng/mL) | 0.220 ± 0.256 | 2.01 ± 4.07 | 0.037 * |
| Parameters | Survivors (n = 34) | Non-Survivors (n = 20) | p Value |
|---|---|---|---|
| Leukocytes (×103/μL) | 13.48 ± 25.44 | 13.19 ± 6.34 | 0.960 |
| Neutrophil percentage (%) | 73.95 ± 17.19 | 84.72 ± 18.89 | 0.041 * |
| Lymphocyte percentage (%) | 16.79 ± 11.35 | 7.48 ± 5.65 | 0.001 * |
| Monocyte percentage (%) | 5.96 ± 2.54 | 3.35 ± 1.42 | 0.001 * |
| Band cells (%) | 0.000 ± 0.000 | 0.08 ± 0.358 | 0.217 |
| Eosinophil percentage (%) | 0.739 ± 0.974 | 0.110 ± 0.281 | 0.007 * |
| Basophil percentage (%) | 0.429 ± 1.23 | 0.085 ± 0.123 | 0.221 |
| Neutrophils (×103/μL) | 7.39 ± 4.44 | 11.93 ± 5.99 | 0.003 * |
| Lymphocytes (×103/μL) | 2.09 ± 4.40 | 0.750 ± 0.426 | 0.181 |
| Monocytes (×103/μL) | 0.519 ± 0.256 | 0.450 ± 0.276 | 0.364 |
| Eosinophils (×103/μL) | 0.096 ± 0.272 | 0.015 ± 0.049 | 0.193 |
| Basophils (×103/μL) | 0.339 ± 1.87 | 0.000 ± 0.000 | 0.423 |
| Erythrocyte (×106/μL) | 4.71 ± 0.893 | 4.75 ± 1.09 | 0.884 |
| Hemoglobin (g/dL) | 14.17 ± 2.56 | 14.42 ± 3.11 | 0.756 |
| Hematocrit (%) | 42.40 ± 7.58 | 42.82 ± 9.44 | 0.863 |
| MCV (fL) | 90.54 ± 5.88 | 91.35 ± 4.15 | 0.597 |
| MCH (pg) | 30.34 ± 2.73 | 30.42 ± 1.64 | 0.905 |
| RDW (%) | 15.07 ± 3.38 | 14.72 ± 2.07 | 0.685 |
| Platelets (×103/μL) | 266.61 ± 111.11 | 240.25 ± 113.83 | 0.416 |
| Parameters | AUC | CI 95% |
|---|---|---|
| Total leukocyte count | 0.702 | 0.557–0.822 |
| Neutrophil count | 0.746 | 0.605–0.857 |
| Lymphocyte count | 0.735 | 0.593–0.849 |
| Monocyte count | 0.605 | 0.458–0.739 |
| D-dimer | 0.730 | 0.548–0.869 |
| Ferritin | 0.777 | 0.601–0.901 |
| CRP | 0.750 | 0.569–0.884 |
| Troponin I | 0.656 | 0.464–0.816 |
| LDH | 0.758 | 0.618–0.867 |
| Procalcitonin | 0.826 | 0.682–0.924 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizo-Téllez, S.A.; Méndez-García, L.A.; Flores-Rebollo, C.; Alba-Flores, F.; Alcántara-Suárez, R.; Manjarrez-Reyna, A.N.; Baltazar-López, N.; Hernández-Guzmán, V.A.; León-Pedroza, J.I.; Zapata-Arenas, R.; et al. The Neutrophil-to-Monocyte Ratio and Lymphocyte-to-Neutrophil Ratio at Admission Predict In-Hospital Mortality in Mexican Patients with Severe SARS-CoV-2 Infection (Covid-19). Microorganisms 2020, 8, 1560. https://doi.org/10.3390/microorganisms8101560
Rizo-Téllez SA, Méndez-García LA, Flores-Rebollo C, Alba-Flores F, Alcántara-Suárez R, Manjarrez-Reyna AN, Baltazar-López N, Hernández-Guzmán VA, León-Pedroza JI, Zapata-Arenas R, et al. The Neutrophil-to-Monocyte Ratio and Lymphocyte-to-Neutrophil Ratio at Admission Predict In-Hospital Mortality in Mexican Patients with Severe SARS-CoV-2 Infection (Covid-19). Microorganisms. 2020; 8(10):1560. https://doi.org/10.3390/microorganisms8101560
Chicago/Turabian StyleRizo-Téllez, Salma A., Lucia A. Méndez-García, Cruz Flores-Rebollo, Fernando Alba-Flores, Raúl Alcántara-Suárez, Aarón N. Manjarrez-Reyna, Neyla Baltazar-López, Verónica A. Hernández-Guzmán, José I. León-Pedroza, Rogelio Zapata-Arenas, and et al. 2020. "The Neutrophil-to-Monocyte Ratio and Lymphocyte-to-Neutrophil Ratio at Admission Predict In-Hospital Mortality in Mexican Patients with Severe SARS-CoV-2 Infection (Covid-19)" Microorganisms 8, no. 10: 1560. https://doi.org/10.3390/microorganisms8101560