Copper Precipitation Behavior during Continuous Cooling and Subsequent Aging of Powder-Forged Fe-2.5Cu-C Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Dilatometric Curves and Microstructure of Specimens Cooled at Different Rates
3.2. Microstructures of the Specimens Cooled at Different Rates
3.3. Microstructures of the Specimens after Subsequent Aging
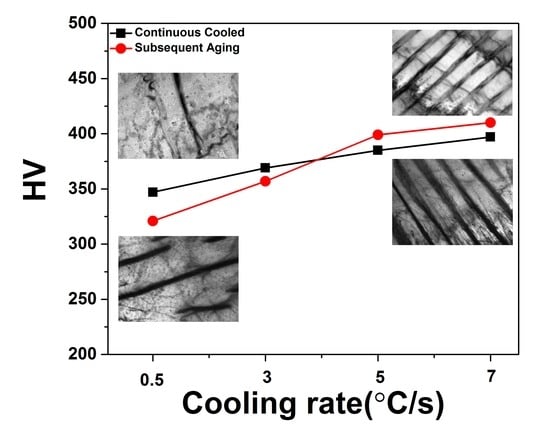
3.4. Hardness of the Specimens during Continuous Cooling and Subsequent Aging
4. Discussion
4.1. Evolution of Copper Precipitates during Different Cooling Rates and Subsequent Aging
4.2. Contribution of the Copper Precipitates to the Strength of the Alloys
5. Conclusions
- (1)
- For the Fe-2.5Cu-C alloy, the powder-forged connecting rods should be cooled not higher than 7 °C/s to obtain a pearlite structure. When the cooling rate was less than 7 °C/s, copper precipitates were consistent with the interphase mechanism, while when the cooling rate was higher than 7 °C/s, copper was supersaturated in the matrix;
- (2)
- During the subsequent aging process, precipitates would grow especially for those located in the ferrite matrix. In the specimen cooled at 7 °C/s, BCC copper precipitates formed after aging;
- (3)
- The growth of copper precipitates would decrease the hardness of specimens cooled less than 4 °C/s. Aging would further improve the hardness of the specimens cooled at rates faster than 4 °C/s because the precipitate strengthening is stronger than the precipitate coarsening effect.
Author Contributions
Funding
Conflicts of Interest
References
- Ilia, E.; Plamondon, P.; Masse, J.P.; L’Espérance, G. Copper precipitation at engine operating temperatures in powder-forged connecting rods manufactured with Fe-Cu-C alloys. Mater. Sci. Eng. A 2019, 767, 138383. [Google Scholar] [CrossRef]
- Ilia, E.; O’Neill, M.; Tutton, K.; Lanni, G.; Letourneau, S.; Haehnel, M. Benchmarking the industry: Powder forging makes a better connecting rod. In SAE Tranactions; SAE International: Warrendale, PA, USA, 2005; pp. 340–352. [Google Scholar]
- Suzuki, H.; Sawayama, T.; Ilia, E.; Tutton, K. New material with improved machinability and strength for powder forged connecting rods. In SAE Tranactions; SAE International: Warrendale, PA, USA, 2006; pp. 556–562. [Google Scholar]
- Brown, G. Development of alloy systems for powder forging. Met. Technol. 1976, 3, 229–236. [Google Scholar] [CrossRef]
- Dorofeyev, Y.; Dorofeyev, V. Powder forging in PSRSPU. Met. Powder Rep. 2018, 73, 87–93. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Wang, H.L.; Liu, F.P.; Yao, W.J.; Jiang, F.; Sun, J.; Wang, F.Y. Effects of copper content on microstructure and mechanical properties of powder-forged rod Fe-C-Cu alloys manufactured at elevated temperature. Mater. Sci. Eng. A 2019, 743, 197–206. [Google Scholar] [CrossRef]
- Thompson, S.; Krauss, G.; Tseng, C.-C. A new model of interphase precipitation in copper-containing steels. J. Mater. Sci. Lett. 1998, 17, 2075–2078. [Google Scholar] [CrossRef]
- Thompson, S.; Krauss, G. Copper precipitation during continuous cooling and isothermal aging of A710-type steels. Metall. Mater. Trans. A 1996, 27, 1573–1588. [Google Scholar] [CrossRef]
- Thompson, S.; Colvin, D.; Krauss, G. Austenite decomposition during continuous cooling of an HSLA-80 plate steel. Metall. Mater. Trans. A 1996, 27, 1557–1571. [Google Scholar] [CrossRef]
- Kimura, Y.; Takaki, S. Phase transformation mechanism of Fe-Cu alloys. ISIJ Int. 1997, 37, 290–295. [Google Scholar] [CrossRef]
- Shi, X.; Yan, W.; Wang, W.; Yang, Z.; Shan, Y.; Yang, K. Dynamic continuous cooling transformation behavior of a novel Cu-bearing pipeline steel. ISIJ Int. 2016, 56, 2284–2289. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Li, C.; Gu, J.; Liu, W. Direct observation of Cu interphase precipitation in continuous cooling transformation by atom probe tomography. Philos. Mag. 2014, 94, 306–315. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, W.; Xiong, X. Correlation of Cu precipitation with austenite—Ferrite transformation in a continuously cooled multicomponent steel: An atom probe tomography study. J. Mater. Res. 2012, 27, 1060–1067. [Google Scholar] [CrossRef]
- Monzen, R.; Jenkins, M.; Sutton, A. The bcc-to-9R martensitic transformation of Cu precipitates and the relaxation process of elastic strains in an Fe-Cu alloy. Philos. Mag. A 2000, 80, 711–723. [Google Scholar] [CrossRef]
- Monzen, R.; Iguchi, M.; Jenkins, M. Structural changes of 9R copper precipitates in an aged Fe-Cu alloy. Philos. Mag. Lett. 2000, 80, 137–148. [Google Scholar] [CrossRef]
- Chairuangsri, T.; Edmonds, D. The precipitation of copper in abnormal ferrite and pearlite in hyper-eutectoid steels. Acta Mater. 2000, 48, 3931–3949. [Google Scholar] [CrossRef]
- Maruyama, N.; Sugiyama, M.; Hara, T.; Tamehiro, H. Precipitation and phase transformation of copper particles in low alloy ferritic and martensitic steels. Mater. Trans. JIM 1999, 40, 268–277. [Google Scholar] [CrossRef] [Green Version]
- Fourlaris, G.; Baker, A.; Papadimitriou, G. Microscopic characterisation of ε-Cu interphase precipitation in hypereutectoid Fe-C-Cu alloys. Acta Metall. Et Mater. 1995, 43, 2589–2604. [Google Scholar] [CrossRef]
- Heo, Y.-U.; Kim, Y.K.; Kim, J.S.; Kim, J.K. Phase transformation of Cu precipitates from bcc to fcc in Fe-3Si-2Cu alloy. Acta Mater. 2013, 61, 519–528. [Google Scholar] [CrossRef]
- Lozano-Perez, S.; Sha, G.; Titchmarsh, J.M.; Jenkins, M.L.; Hirosawa, S.; Cerezo, A.; Smith, G.D.W. Comparison of the number densities of nanosized Cu-rich precipitates in ferritic alloys measured using EELS and EDX mapping, HREM and 3DAP. J. Mater. Sci. 2006, 41, 2559–2565. [Google Scholar] [CrossRef]
- Lozano-Perez, S.; Jenkins, M.; Titchmarsh, J. Evidence for deformation-induced transformations of Cu-rich precipitates in an aged FeCu alloy. Philos. Mag. Lett. 2006, 86, 367–374. [Google Scholar] [CrossRef]
- Isheim, D.; Gagliano, M.S.; Fine, M.E.; Seidman, D.N. Interfacial segregation at Cu-rich precipitates in a high-strength low-carbon steel studied on a sub-nanometer scale. Acta Mater. 2006, 54, 841–849. [Google Scholar] [CrossRef]
- Liu, Q.-D.; Zhao, S.-J. Comparative study on austenite decomposition and Cu precipitation during continuous cooling transformation. Metall. Mater. Trans. A 2013, 44, 163–171. [Google Scholar] [CrossRef]
- Jung, J.G.; Jung, M.; Lee, S.M.; Shin, E.; Shin, H.C.; Lee, Y.K. Cu precipitation kinetics during martensite tempering in a medium C steel. J. Alloy. Compd. 2013, 553, 299–307. [Google Scholar] [CrossRef]
- Yang, J.B.; Yamashita, T.; Sano, N.; Enomoto, M. Simulation of competitive Cu precipitation in steel during non-isothermal aging. Mater. Ence Eng. A 2008, 487, 128–136. [Google Scholar] [CrossRef]
- Han, G.; Xie, Z.J.; Li, Z.Y.; Lei, B.; Shang, C.J.; Misra, R.D.K. Evolution of crystal structure of Cu precipitates in a low carbon steel. Mater. Des. 2017, 135, 92–101. [Google Scholar] [CrossRef]
- Xi, T.; Shahzad, M.B.; Xu, D.; Zhao, J.; Yang, C.; Qi, M.; Yang, K. Copper precipitation behavior and mechanical properties of Cu-bearing 316L austenitic stainless steel: A comprehensive cross-correlation study. Mater. Sci. Eng. A 2016, 675, 243–252. [Google Scholar] [CrossRef]
- Takahashi, J.; Kawakami, K.; Kobayashi, Y. Consideration of particle-strengthening mechanism of copper-precipitation-strengthened steels by atom probe tomography analysis. Mater. Sci. Eng. A 2012, 535, 144–152. [Google Scholar] [CrossRef]
- Chen, Z.; Kioussis, N.; Ghoniem, N. Influence of nanoscale Cu precipitates in α-Fe on dislocation core structure and strengthening. Phys. Rev. B 2009, 80, 184104. [Google Scholar] [CrossRef] [Green Version]
- Kolli, R.P.; Seidman, D.N. The temporal evolution of the decomposition of a concentrated multicomponent Fe–Cu-based steel. Acta Mater. 2008, 56, 2073–2088. [Google Scholar] [CrossRef]
- Charleux, M.; Livet, F.; Bley, F.; Louchet, F.; Bréchet, Y. Thermal ageing of an Fe-Cu alloy: Microstructural evolution and precipitation hardening. Philos. Mag. A 1996, 73, 883–897. [Google Scholar] [CrossRef]
- Youle, A.; Ralph, B. A Study of the precipitation of copper from α-iron in the pre-peak to peak hardness range of ageing. Met. Sci. J. 1972, 6, 149–152. [Google Scholar] [CrossRef]
- Eto, F.H. Development of fracture splitting connecting rod. JSAE Rev. 2002, 23, 101–104. [Google Scholar]
- Kondo, Y. Behaviour of copper during high temperature oxidation of steel containing copper. ISIJ Int. 2004, 44, 1576–1580. [Google Scholar] [CrossRef]
- Krielaart, G.P.; Sietsma, J.; van der Zwaag, S. Ferrite formation in Fe-C alloys during austenite decomposition under non-equilibrium interface conditions. Mater. Sci. Eng. A 1997, 237, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Khalid, F.; Edmonds, D. A transmission electron microscopy study of copper precipitation in the cementite phase of hypereutectoid alloy steels. Metall. Trans. A 1993, 24, 781–793. [Google Scholar] [CrossRef]
- Misra, R.; Jia, Z.; O’Malley, R.J.; Jansto, S. Precipitation behavior during thin slab thermomechanical processing and isothermal aging of copper-bearing niobium-microalloyed high strength structural steels: The effect on mechanical properties. Mater. Sci. Eng. A 2011, 528, 8772–8780. [Google Scholar] [CrossRef]
- Fine, M.; Isheim, D. Origin of copper precipitation strengthening in steel revisited. Scr. Mater. 2005, 53, 115–118. [Google Scholar] [CrossRef]
- Deschamps, A.; Militzer, M. Precipitation kinetics and strengthening of a Fe-0.8 wt.% Cu alloy. ISIJ Int. 2001, 41, 196–205. [Google Scholar] [CrossRef]
- Russell, K.C.; Brown, L. A dispersion strengthening model based on differing elastic moduli applied to the iron-copper system. Acta Metall. 1972, 20, 969–974. [Google Scholar] [CrossRef]













| Elements (wt. %) | Cu | C | Mn | S | Fe |
|---|---|---|---|---|---|
| Fe-2.5Cu-C | 2.52 | 0.61 | 0.49 | 0.12 | Bal. |
| Cooling rates (°C/s) | Vf (%) | Sp (nm) | dp (μm) | HV |
|---|---|---|---|---|
| 0.5 | 23 | 380 | 139 | 347 |
| 3 | 8 | 263 | 102 | 369 |
| 5 | 6 | 179 | 82 | 385 |
| 7 | 2 | 117 | 61 | 397 |
| Cooling Rates (°C/s) | Average Diameter (nm) | Volume Fraction | ||
|---|---|---|---|---|
| Before Aging | After Aging | Before Aging | After Aging | |
| 0.5 | 12 | 21 | 1.2% | 2.15% |
| 7 | 0 | 10 | 0 | 1% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wu, Y.; Zhang, T.; Jiang, F. Copper Precipitation Behavior during Continuous Cooling and Subsequent Aging of Powder-Forged Fe-2.5Cu-C Alloy. Metals 2020, 10, 1350. https://doi.org/10.3390/met10101350
Wang S, Wu Y, Zhang T, Jiang F. Copper Precipitation Behavior during Continuous Cooling and Subsequent Aging of Powder-Forged Fe-2.5Cu-C Alloy. Metals. 2020; 10(10):1350. https://doi.org/10.3390/met10101350
Chicago/Turabian StyleWang, Sui, Yake Wu, Tengyu Zhang, and Feng Jiang. 2020. "Copper Precipitation Behavior during Continuous Cooling and Subsequent Aging of Powder-Forged Fe-2.5Cu-C Alloy" Metals 10, no. 10: 1350. https://doi.org/10.3390/met10101350