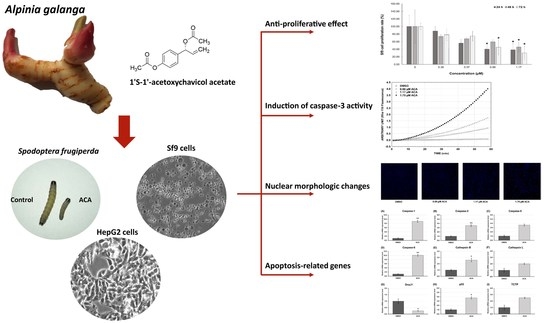
A Novel Insecticidal Molecule Extracted from Alpinia galanga with Potential to Control the Pest Insect Spodoptera frugiperda
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Extraction and Isolation of 1′S-1′-Acetoxychavicol Acetate
2.3. Insect Rearing and Insecticide Treatments
2.4. Cell Culture
2.5. Cell Viability Assay
2.6. Cell Proliferation Assay
2.7. Caspase-3 Activity Assay
2.8. Hoechst 33342 Staining
2.9. Cell Cycle Analysis
2.10. RNA Extraction, cDNA Synthesis and Real-Time Quantitative PCR (RT-qPCR)
2.11. Statistical Analysis
3. Results
3.1. Extract Yield
3.2. Effects of ACA on S. frugiperda Larvae
3.3. Cytotoxicity of ACA on Sf9 and HepG2 Cells
3.4. Sf9 Cell Proliferation Is Inhibited by ACA
3.5. Gene Expression Profile of Apoptosis-Related Genes after ACA Treatment of Sf9 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chormule, A.; Shejawal, N.; Sharanabasappa; Kalleshwaraswamy, C.; Asokan, R.; Swamy, H.M. First report of the fall Armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera, Noctuidae) on sugarcane and other crops from Maharashtra, India. J. Entomol. Zool. Stud. 2019, 7, 114–117. [Google Scholar]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamo, M. First Report of Outbreaks of the Fall Armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a New Alien Invasive Pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murúa, M.G.; Vera, M.T.; Abraham, S.; Juarçz, M.L.; Prieto, S.; Head, G.P.; Willink, E. Fitness and Mating Compatibility of Spodoptera frugiperda (Lepidoptera: Noctuidae) Populations from Different Host Plant Species and Regions in Argentina. Ann. Entomol. Soc. Am. 2008, 101, 639–649. [Google Scholar] [CrossRef] [Green Version]
- Mota-Sanchez, D.; Wise, J.C. The Arthropod Pesticide Resistance Database; Michigan State University: East Lansing, MI, USA, 2008. [Google Scholar]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. Production of plant secondary metabolites: A historical perspective. Plant Sci. 2001, 161, 839–851. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical Insecticides in the Twenty-First Century-Fulfilling Their Promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef] [Green Version]
- Anirban, C.; Santanu, P. A Review on Phytochemical and Pharmacological Potential of Alpinia galanga. Pharmacogn. J. 2018, 10, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Ito, K.; Nakazato, T.; Murakami, A.; Yamato, K.; Miyakawa, Y.; Yamada, T.; Hozumi, N.; Ohigashi, H.; Ikeda, Y.; Kizaki, M. Induction of apoptosis in human myeloid leukemic cells by 1′-acetoxychavicol acetate through a mitochondrial- and Fas-mediated dual mechanism. Clin. Cancer Res. 2004, 10, 2120–2130. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, F.; Subramanian, P.; Ibrahim, H.; Abdul Malek, S.N.; Lee, G.S.; Hong, S.L. Chemical composition, antifeedant, repellent, and toxicity activities of the rhizomes of galangal, Alpinia galanga against Asian subterranean termites, Coptotermes gestroi and Coptotermes curvignathus (Isoptera: Rhinotermitidae). J. Insect Sci. 2015, 15, 175. [Google Scholar] [CrossRef]
- Datta, R.; Kaur, A.; Saraf, I.; Singh, I.P.; Kaur, S. Effect of crude extracts and purified compounds of Alpinia galanga on nutritional physiology of a polyphagous lepidopteran pest, Spodoptera litura (Fabricius). Ecotoxicol. Environ. Saf. 2019, 168, 324–329. [Google Scholar] [CrossRef]
- Sukhirun, N.; Pluempanupat, W.; Bullangpoti, V.; Koul, O. Bioefficacy of Alpinia galanga (Zingiberaceae) rhizome extracts, (E)-p-acetoxycinnamyl alcohol, and (E)-p-coumaryl alcohol ethyl ether against Bactrocera dorsalis (Diptera: Tephritidae) and the impact on detoxification enzyme activities. J. Econ. Entomol. 2011, 104, 1534–1540. [Google Scholar] [CrossRef] [PubMed]
- Ruttanaphan, T.; Pluempanupat, W.; Aungsirisawat, C.; Boonyarit, P.; Le Goff, G.; Bullangpoti, V. Effect of Plant Essential Oils and Their Major Constituents on Cypermethrin Tolerance Associated Detoxification Enzyme Activities in Spodoptera litura (Lepidoptera: Noctuidae). J. Econ. Entomol. 2019, 112, 2167–2176. [Google Scholar] [CrossRef] [PubMed]
- Poonsri, W.; Pengsook, A.; Pluempanupat, W.; Yooboon, T.; Bullangpoti, V. Evaluation of Alpinia galanga (Zingiberaceae) extracts and isolated trans-cinnamic acid on some mosquitoes larvae. Chem. Biol. Technol. Agric. 2019, 6, 17. [Google Scholar] [CrossRef]
- Pengsook, A.; Puangsomchit, A.; Yooboon, T.; Bullangpoti, V.; Pluempanupat, W. Insecticidal activity of isolated phenylpropanoids from Alpinia galanga rhizomes against Spodoptera litura. Nat. Prod. Res. 2020, 1–5. [Google Scholar] [CrossRef]
- Poitout, S.; Bues, R. Linolenic acid requirements of lepidoptera Noctuidae Quadrifinae Plusiinae: Chrysodeixis chalcites Esp., Autographa gamma L.’ Macdunnoughia confusa Stph., Trichoplusia ni Hbn. reared on artificial diets. Ann. Nutr. Aliment. 1974, 28, 173–187. [Google Scholar] [PubMed]
- Moné, Y.; Nhim, S.; Gimenez, S.; Legeai, F.; Seninet, I.; Parrinello, H.; Negre, N.; d′Alencon, E. Characterization and expression profiling of microRNAs in response to plant feeding in two host-plant strains of the lepidopteran pest Spodoptera frugiperda. BMC Genom. 2018, 19, 804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, S.S.; van Emden, H.F. Growth disruption, abnormalities and mortality of Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae) caused by Azadirachtin. Neotrop. Entomol. 2001, 30, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, Y.; Isman, M.B. Comparative growth inhibitory and antifeedant effects of plant extracts and pure allelochemicals on four phytophagous insect species. J. Appl. Entomol. 2004, 128, 32–38. [Google Scholar] [CrossRef]
- Giraudo, M.; Hilliou, F.; Fricaux, T.; Audant, P.; Feyereisen, R.; Le Goff, G. Cytochrome P450s from the fall armyworm (Spodoptera frugiperda): Responses to plant allelochemicals and pesticides. Insect Mol. Biol. 2015, 24, 115–128. [Google Scholar] [CrossRef]
- Rancurel, C.; van Tran, T.; Elie, C.; Hilliou, F. SATQPCR: Website for statistical analysis of real-time quantitative PCR data. Mol. Cell Probes 2019, 46, 101418. [Google Scholar] [CrossRef]
- Selin-Rani, S.; Senthil-Nathan, S.; Thanigaivel, A.; Vasantha-Srinivasan, P.; Edwin, E.S.; Ponsankar, A.; Lija-Escaline, J.; Kalaivani, K.; Abdel-Megeed, A.; Hunter, W.B.; et al. Toxicity and physiological effect of quercetin on generalist herbivore, Spodoptera litura Fab. and a non-target earthworm Eisenia fetida Savigny. Chemosphere 2016, 165, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Vieira Ribeiro, A.; de Sá Farias, E.; Almeida Santos, A.; Andrade Filomeno, C.; Barbosa dos Santos, I.; Almeida Barbosa, L.C.; Coutinho Picanço, M. Selection of an essential oil from Corymbia and Eucalyptus plants against Ascia monuste and its selectivity to two non-target organisms. Crop Prot. 2018, 110, 207–213. [Google Scholar] [CrossRef]
- Casida, J.E. The greening of pesticide-environment interactions: Some personal observations. Environ. Health Perspect. 2012, 120, 487–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knasmuller, S.; Parzefall, W.; Sanyal, R.; Ecker, S.; Schwab, C.; Uhl, M.; Mersch-Sundermann, V.; Williamson, G.; Hietsch, G.; Langer, T.; et al. Use of metabolically competent human hepatoma cells for the detection of mutagens and antimutagens. Mutat. Res. 1998, 402, 185–202. [Google Scholar] [CrossRef]
- Thrift, R.N.; Forte, T.M.; Cahoon, B.E.; Shore, V.G. Characterization of lipoproteins produced by the human liver cell line, Hep G2, under defined conditions. J. Lipid. Res. 1986, 27, 236–250. [Google Scholar]
- Acosta, D.; Sorensen, E.M.; Anuforo, D.C.; Mitchell, D.B.; Ramos, K.; Santone, K.S.; Smith, M.A. An in vitro approach to the study of target organ toxicity of drugs and chemicals. Vitr. Cell Dev. Biol. 1985, 21, 495–504. [Google Scholar] [CrossRef]
- Dehn, P.F.; Allen-Mocherie, S.; Karek, J.; Thenappan, A. Organochlorine insecticides: Impacts on human HepG2 cytochrome P4501A, 2B activities and glutathione levels. Toxicol. Vitr. 2005, 19, 261–273. [Google Scholar] [CrossRef]
- Yun, X.; Huang, Q.; Rao, W.; Xiao, C.; Zhang, T.; Mao, Z.; Wan, Z. A comparative assessment of cytotoxicity of commonly used agricultural insecticides to human and insect cells. Ecotoxicol. Environ. Saf. 2017, 137, 179–185. [Google Scholar] [CrossRef]
- Davis, R.E.; Kelly, T.J.; Masler, E.P.; Fescemyer, H.W.; Thyagaraja, B.S.; Borkovec, A.B. Hormonal control of vitellogenesis in the gypsy moth, Lymantria dispar (L.): Suppression of haemolymph vitellogenin by the juvenile hormone analogue, methoprene. J. Insect Physiol. 1990, 36, 231–238. [Google Scholar] [CrossRef]
- Merzendorfer, H. Chitin synthesis inhibitors: Old molecules and new developments. Insect Sci. 2013, 20, 121–138. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Minakuchi, C.; Ueno, T. Inhibition of [(3)H] ponasterone a binding by ecdysone agonists in the intact Sf-9 cell line. Steroids 2000, 65, 537–542. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Sun, Z.; Xie, J.; Zhong, G.; Yi, X. Azadirachtin induced apoptosis in the prothoracic gland in Bombyx mori and a pronounced Ca(2+) release effect in Sf9 cells. Int. J. Biol. Sci. 2017, 13, 1532–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, B.; Zhang, J.; Cui, G.; Sun, R.; Yi, X.; Zhong, G. Azadirachtin Affects the Growth of Spodoptera litura Fabricius by Inducing Apoptosis in Larval Midgut. Front. Physiol. 2018, 9, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, B.; Zhang, J.; Jiang, Z.; Cui, G.; Veeran, S.; Zhong, G. Harmine induced apoptosis in Spodoptera frugiperda Sf9 cells by activating the endogenous apoptotic pathways and inhibiting DNA topoisomerase I activity. Pestic Biochem. Physiol. 2019, 155, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Shu, B.; Zhang, J.; Sethuraman, V.; Cui, G.; Yi, X.; Zhong, G. Transcriptome analysis of Spodoptera frugiperda Sf9 cells reveals putative apoptosis-related genes and a preliminary apoptosis mechanism induced by azadirachtin. Sci. Rep. 2017, 7, 13231. [Google Scholar] [CrossRef]
- Cooper, D.M.; Granville, D.J.; Lowenberger, C. The insect caspases. Apoptosis 2009, 14, 247–256. [Google Scholar] [CrossRef]
- Lord, C.E.; Gunawardena, A.H. Programmed cell death in C. elegans, mammals and plants. Eur. J. Cell Biol. 2012, 91, 603–613. [Google Scholar] [CrossRef]
- Courtiade, J.; Pauchet, Y.; Vogel, H.; Heckel, D.G. A comprehensive characterization of the caspase gene family in insects from the order Lepidoptera. BMC Genom. 2011, 12, 357. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Li, Z.Q.; Ou-Yang, Y.Y.; Huang, G.H. Identification of four caspase genes from Spodoptera exigua (Lepidoptera: Noctuidae) and their regulations toward different apoptotic stimulations. Insect Sci. 2019. [Google Scholar] [CrossRef]
- Huang, J.; Lv, C.; Hu, M.; Zhong, G. The mitochondria-mediate apoptosis of Lepidopteran cells induced by azadirachtin. PLoS ONE 2013, 8, e58499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Cheng, X.; Meng, Q.; Wang, P.; Shu, B.; Hu, Q.; Hu, M.; Zhong, G. Azadirachtin-induced apoptosis involves lysosomal membrane permeabilization and cathepsin L release in Spodoptera frugiperda Sf9 cells. Int. J. Biochem. Cell Biol. 2015, 64, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Shu, B.; Wang, W.; Hu, Q.; Huang, J.; Hu, M.; Zhong, G. A comprehensive study on apoptosis induction by azadirachtin in Spodoptera frugiperda cultured cell line Sf9. Arch. Insect Biochem. Physiol. 2015, 89, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Chen, A.; Gobert, V.; Auge, B.; Beau, M.; Burlet-Schiltz, O.; Haenlin, M.; Waltzer, L. Control of RUNX-induced repression of Notch signaling by MLF and its partner DnaJ-1 during Drosophila hematopoiesis. PLoS Genet. 2017, 13, e1006932. [Google Scholar] [CrossRef] [PubMed]
- Tsou, W.L.; Ouyang, M.; Hosking, R.R.; Sutton, J.R.; Blount, J.R.; Burr, A.A.; Todi, S.V. The deubiquitinase ataxin-3 requires Rad23 and DnaJ-1 for its neuroprotective role in Drosophila melanogaster. Neurobiol. Dis. 2015, 82, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Shu, B.; Jia, J.; Zhang, J.; Sethuraman, V.; Yi, X.; Zhong, G. DnaJ homolog subfamily A member1 (DnaJ1) is a newly discovered anti-apoptotic protein regulated by azadirachtin in Sf9 cells. BMC Genom. 2018, 19, 413. [Google Scholar] [CrossRef] [Green Version]
- Guan, F.; Zhang, J.; Shen, H.; Wang, X.; Padovan, A.; Walsh, T.K.; Tay, W.T.; Gordon, K.H.J.; James, W.; Czepak, C.; et al. Whole-genome sequencing to detect mutations associated with resistance to insecticides and Bt proteins in Spodoptera frugiperda. Insect Sci. 2020. [Google Scholar] [CrossRef]





| Name | Primer Sequence | Fragment Length (bp) | PCR Efficiencies (%) |
|---|---|---|---|
| Sf-Caspase-1-F | 5′-AATGCTGGACGGAAAACAAG-3′ | 139 | 99 |
| Sf-Caspase-1-R | 5′-AACTGGCATCCTAGCGACAC-3′ | ||
| Sf-Caspase-2-F | 5′-TCCAGTCCACCCTGATTTTC-3′ | 83 | 103 |
| Sf-Caspase-2-R | 5′-ACCAAGATCCACGTTTACGG-3′ | ||
| Sf-Caspase-5-F | 5′-GGCCTCTACGAGTGATGGAC-3′ | 89 | 99 |
| Sf-Caspase-5-R | 5′-CGGAAGACACGTCAGTCAAA-3′ | ||
| Sf-Caspase-6-F | 5′-ACCACAAGGAATGGAAGTGG-3′ | 149 | 101 |
| Sf-Caspase-6-R | 5′-GTGCTGTGTCCGGTACTTCA-3′ | ||
| Sf-Cathepsin B-F | 5′-AACGGTGACTCCAAAACACC-3′ | 98 | 109 |
| Sf-Cathepsin B-R | 5′-GAGTACACGTGCTTGCCGTA-3′ | ||
| Sf-Cathepsin L-F | 5′-AGTGCAGGTACAACCCCAAG-3′ | 146 | 100 |
| Sf-Cathepsin L-R | 5′-CTGGAAGGTCTCCTGTGAGG-3′ | ||
| Sf-DnaJ1-F | 5′-TGAGAGAGGGAGGAGTTGGA-3′ | 93 | 95 |
| Sf-DnaJ1-R | 5′-GTCTACGACCACCGCTGAAT-3′ | ||
| Sf-p53-F | 5′-GCACTTGATATCGGTGGAGAA-3′ | 150 | 96 |
| Sf-p53-R | 5′-GATCCTACAGTCACCCAGCA-3′ | ||
| Sf-TCTP-F | 5′-GGACATCCTTGGCAGGTTTA-3′ | 147 | 105 |
| Sf-TCTP-R | 5′-TCCTCCTCAAGACCATGCTT-3′ | ||
| G6PD-F | 5′-GGCCCTGTGGCTAACAGAAT-3′ | 142 | 98 |
| G6PD-R | 5′-CATCGTCTCTACCAAAAGGCTTC-3′ | ||
| L18-F | 5′-CGTATCAACCGACCTCCACT-3′ | 126 | 108 |
| L18-R | 5′-AGGCACCTTGTAGAGCCTCA-3′ | ||
| RpL4-F | 5′-CAACAAGAGGGGTTCACGAT-3′ | 149 | 98 |
| RpL4-R | 5′-GCACGATCAGTTCGGGTATC-3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruttanaphan, T.; de Sousa, G.; Pengsook, A.; Pluempanupat, W.; Huditz, H.-I.; Bullangpoti, V.; Le Goff, G. A Novel Insecticidal Molecule Extracted from Alpinia galanga with Potential to Control the Pest Insect Spodoptera frugiperda. Insects 2020, 11, 686. https://doi.org/10.3390/insects11100686
Ruttanaphan T, de Sousa G, Pengsook A, Pluempanupat W, Huditz H-I, Bullangpoti V, Le Goff G. A Novel Insecticidal Molecule Extracted from Alpinia galanga with Potential to Control the Pest Insect Spodoptera frugiperda. Insects. 2020; 11(10):686. https://doi.org/10.3390/insects11100686
Chicago/Turabian StyleRuttanaphan, Torranis, Georges de Sousa, Anchulee Pengsook, Wanchai Pluempanupat, Hannah-Isadora Huditz, Vasakorn Bullangpoti, and Gaëlle Le Goff. 2020. "A Novel Insecticidal Molecule Extracted from Alpinia galanga with Potential to Control the Pest Insect Spodoptera frugiperda" Insects 11, no. 10: 686. https://doi.org/10.3390/insects11100686