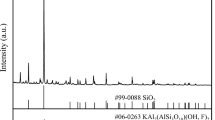
Abstract
The objective of this study was to investigate thiourea leaching of gold present in the uranium tailings. Sulfuric acid treatment for raw material is important in order to dissolve uranium as well as to remove easily leachable metals. The acidic thiourea solution was proposed as a non-toxic possible alternative process route to cyanide in gold dissolution. The acidic system of thiourea-sodium sulfite was studied in the gold dissolution process. The presence of sodium sulfite can significantly reduce thiourea decomposition, accelerate the gold dissolution process, decrease the leaching time, and decrease the activation energy of gold dissolution. The activation energy for gold dissolution was evaluated. The activation energy solution with sodium sulfite is 7.62 kJ mol−1 which is much lower than 9.32 kJ mol−1 in the absence of sodium sulfite. Low activation energy confirms the surface reaction dissolution mechanism. This method demonstrates the possibility of extracting gold directly from low-grade ores without any physical or chemical pre-concentration method with a more environmentally friendly process.
Graphical Abstract


















Similar content being viewed by others
References
Li H, Long H, Zhang L, Yin S, Li S, Zhu F, Xie H (2020) Effectiveness of microwave-assisted thermal treatment in the extractiosn of gold in cyanide tailings. J Hazard Mater 384:121456. https://doi.org/10.1016/j.jhazmat.2019.121456
Hilson G, Monhemius AJ (2006) Alternatives to cyanide in the gold mining industry: what prospects for the future? J Clean Prod 14:1158–1167. https://doi.org/10.1016/j.jclepro.2004.09.005
Altinkaya P, Liipo J, Kolehmainen E, Haapalainen M, Leikola M, Lundstrom M (2019) Leaching of trace amounts of metals from flotation tailings in cupric chloride solutions. Min Metall Explor 36:335–342. https://doi.org/10.1007/s42461-018-0015-9
Ofori-Sarpong G, Osseo-Asare K (2013) Preg-robbing of gold from cyanide and noncyanide complexes: effect of fungi pretreatment of carbonaceous matter. Int J Miner Process 119:27–33. https://doi.org/10.1016/j.minpro.2012.12.007
Ali S, Ullah S, Haris M, Iqbal Y (2018) Extraction of gold from Boulangrite ore by ammonium thiocyanate (NH4SCN)s. Pak J Sci Ind Res Series A 61:145–148
Li J, Miller JD (2006) A review of gold leaching in acid thiourea solutions. Miner Process Extr Metall Rev 27:177–214. https://doi.org/10.1080/08827500500339315
Li J, Miller JD (2007) Reaction kinetics of gold dissolution in acid thiourea solution using ferric sulfate as oxidant. Hydrometallurgy 89:279–288. https://doi.org/10.1016/j.hydromet.-2007.07.015
Feng D, Van-Deventer JSJ (2007) The role of oxygen in thiosulphate leaching of gold. Hydrometallurgy 85:193–202. https://doi.org/10.1016/j.hydromet.2006.09.003
Deng T, Liao M (2002) Gold recovery enhancement from a refractory flotation concentrate by sequential bioleaching and thiourea leach. Hydrometallurgy 63:249–255. https://doi.org/10.1016/S0304-386X(01)00226-2
Boboev IR, Kurbonov SK, Selnitsyn RS (2019) Use of thiourea leaching during gold-containing dump treatment. Metallurgist 63:633–641. https://doi.org/10.1007/s11015-019-00869-w
Akcil A, Erust C, Gahan CS, Ozgun M, Sahin M, Tuncuk A (2015) Precious metal recovery from waste printed circuit boards using cyanide and non-cyanide lixiviants a review. Waste Manag 45:258–271. https://doi.org/10.1016/j.wasman.2015.01.017
Chai LY, Wang YY (2007) Mechanism of gold dissolving in alkaline thiourea solution. J Central South Univ Technol. https://doi.org/10.1007/s11771-007-0094-0
Guo Y, Guo X, Wu H, Li S, Wang G, Liu X, Wang D (2017) A novel bio oxidation and two-step thiourea leaching method applied to a refractory gold concentrate. Hydrometallurgy 171:213–221. https://doi.org/10.1016/j.hydromet.2017.05.023
Vanegas C, Munoz F (2017) Cyanidation tests and pretreatments to recover gold from a refractory ore in Colombia. Miner Metall Process 34:160–160. https://doi.org/10.19150/mmp.7621
Mohammed HS, Abdel-Monem YK, El-Feky MG, Omer SA, Ahmed MR (2019) Leaching of El-Missikat low-grade fluoritized uranium ore by sulfuric acid: mechanism and kinetic. J Radioanal Nucl Chem 319:245–255. https://doi.org/10.1007/s10967-018-6289-z
Mohammed H, Sadeek S, Mahmoud AR, Zaky D (2016) Comparison of AAS, EDXRF, ICP-MS and INAA performance for determination of selected heavy metals in HFO ashes. Microchem J 128:1–6. https://doi.org/10.1016/j.microc.2016.04.002
Mohammed H, Sadeek S, Mahmoud AR (2016) Accurate determination of uranium and thorium in Egyptian oil ashes. Microchem J 124:699–702. https://doi.org/10.1016/j.microc.2015.10.034
Mohammed H, Sadeek S, Mahmoud AR, Diab H, Zaky D (2016) Natural radioactivity and radiological hazard assessment of Egyptian oil ashes. Environ Sci Pollut Res 23:15584–15592. https://doi.org/10.1007/s11356-016-6736-8
Liang-Cheng ZO, Jing-Gong WG, Xing LI, Zhang S, Zhang ZF, Ming-Yue HU, Qing-Xue LU (2018) Application of inductively coupled plasma-atomic emission spectrometry/mass spectrometry to phase analysis of gold in gold ores. Chin J Anal Chem 46:e1801–e1809. https://doi.org/10.1016/S1872-2040(17)61070-3
Pyrzynska K (2005) Recent developments in the determination of gold by atomic spectrometry techniques. Spectrochim Acta Part B 60:1316–1322. https://doi.org/10.1016/j.sab.2005.06.010
Ammar F, Omar S, El-Sawey E (2016) Genetic affiliation of gold and uranium mineralization in El-Missikat granite, Central Eastern Desert, Egypt. Nucl Sci Sci J 5:33–47. https://doi.org/10.21608/nssj.2016.30823
Adams MD (ed.) (2016) Gold ore processing: project development and operations, Vol. 15, Elsevier
Bolzan AE, Iwasita T, Arvia AJ (2003) In situ FTIRRAS study of the electro oxidation reactions of thiourea and gold in aqueous acid solutions. J Electroanal Chem 554:49–60. https://doi.org/10.1016/S0022-0728(03)00050-0
Tremblay L, Deschenes G, Ghali E, Mcmullen J, Lanouette M (1996) Gold recovery from a sulphide bearing gold ore by percolation leaching with thiourea. Int J Miner Process 48:225–244. https://doi.org/10.1016/S0301-7516(96)00029-4
Habashi F (1967) Kinetics and mechanism of gold and silver dissolution in cyanide solution. Montana College of Mineral Science and Technology, Butte
Gaspar V, Mejerovich AS, Meretukov MA, Schmiedl J (1994) Practical application of potential-pH diagrams for Au-CS (NH2)2-H2O and Ag-CS (NH2)2-H2O systems for leaching gold and silver with acidic thiourea solution. Hydrometallurgy 34:369–381. https://doi.org/10.1016/0304-386X(94)90073-6
Deng TL, Liao MX, Wang MH, Chen YW, Belzile N (2001) Enhancement of gold extraction from biooxidation residues using an acidic sodium sulphite-thiourea system. Miner Eng 14:263–268. https://doi.org/10.1016/S0892-6875(00)00181-3
Schulze RG (1984) New aspects in thiourea leaching of precious metals. J Metals 36:62–65. https://doi.org/10.1007/BF03338478
Ilyas S, Lee JC (2018) Gold metallurgy and the environment. CRC Press. ISBN 9781138556850
Groenewald T (1976) The dissolution of gold in acidic solutions of thiourea. Hydrometallurgy 1:277–290. https://doi.org/10.1016/0304-386X(76)90004-9
Groenewald T (1977) Potential applications of thiourea in the processing of gold. J S Afr Inst Mining Metall 77:217–223
Deschenes G, Ghali E (1988) Leaching of gold from a chalcopyrite concentrate by thiourea. Hydrometallurgy 20:179–202. https://doi.org/10.1016/0304-386X(88)90051-5
Plaskin IN, Kozhukhova M (1960) Dissolution of gold and silver in solutions of thiourea. Sbornik Nauchnyhk Trudov Institut Tsvetnykh Metallov 33:107–119
Celik H (2004) Extraction of gold and silver from a Turkish gold ore through thiourea leaching. Miner Metall Process 21:144–148. https://doi.org/10.1007/BF03403316
Chai L, Okido M, Wei W (1999) Effect of Na2SO3 on electrochemical aspects of gold dissolution in alkaline thiourea solution. Hydrometallurgy 53:255–266. https://doi.org/10.1016/S0304-386X(99)00051-1
Gabra G (1984) A kinetics study of the leaching of gold from pyrite concentrate using acidified thiourea. In: Kudryk C, Corrigan DA, Liang WW (eds) Precious metals, mining, extraction, and processing, New York: The Metallurgical Society of AIME, pp 145–172
Sparrow GJ, Woodcock JT (1995) Cyanide and other lixiviant leaching systems for gold with some practical applications. Miner Process Extr Metall Rev 14:193–247. https://doi.org/10.1080/08827509508914125
Kai T, Hagiwara T, Haseba H, Takahashi T (1997) Reduction of thiourea consumption in gold extraction by acid thiourea solutions. Ind Eng Chem Res 36:2757–2759. https://doi.org/10.1021/ie970064r
Hiskey B (1984) Thiourea leaching of gold and silver—technology update and additional applications. Mining Metall Explor 1:173–179. https://doi.org/10.1007/BF03402573
Wang Z, Li Y, Ye C (2011) The effect of tri-sodium citrate on the cementation of gold from ferric/thiourea solutions. Hydrometallurgy 110:128–132. https://doi.org/10.1016/j.hydromet.2011.08.011
Hiskey J, Atluri V (1988) Dissolution chemistry of gold and silver in different lixiviants. Miner Process Extr Metall Rev 4:95–134. https://doi.org/10.1080/08827508808952634
Munoz G, Miller J (2000) Noncyanide leaching of an auriferous pyrite ore from Ecuador. Miner Metall Process 17:198–204. https://doi.org/10.1007/BF03402848
Port SN, Horswell SL, Raval R, Schiffrin DJ (1996) Electromodulated Fourier transform infrared reflectance spectroscopy at the gold—aqueous tetramethyl thiourea interface. Langmuir 12:5934–5941. https://doi.org/10.1021/la960346g
Yang X, Moats MS, Miller JD, Wang X, Shi X, Xu H (2011) Thiourea–thiocyanate leaching system for gold. Hydrometallurgy 106:58–63. https://doi.org/10.1016/j.hydromet.2010.11.018
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The contributing editor for this article was João António Labrincha Batista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmed, M.R., Mohammed, H.S., El-Feky, M.G. et al. Gold Leaching Using Thiourea from Uranium Tailing Material, Gabal El-Missikat, Central Eastern Desert, Egypt. J. Sustain. Metall. 6, 599–611 (2020). https://doi.org/10.1007/s40831-020-00295-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-020-00295-2