Abstract
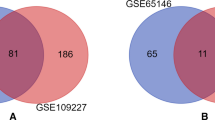
This article was the first attempt to analyze the biological activity of ursodeoxycholic acid in the context of the in silico prediction of changes in activity of genes. Prediction of the dynamics of changes in gene activity was carried out using the DIGEP-Pred algorithm. The obtained gene arrays were analyzed using the GeneMANIA application. Gene annotation was obtained from The Human Protein Atlas. The results showed that the initial sample of genes is distributed according to the localization of expression into several groups: testes, cerebral cortex, placenta, and parathyroid glands. Groups of genes differed among themselves both in the number of members and in the number of relationships between them. The largest group included 25 genes. The activity of the genes of this group was localized to the testes. According to the prediction, all members of this group will be repressed after exposure to ursodeoxycholic acid. The next group consisted of 14 genes located in the cerebral cortex. Within this group, both an increase and a decrease in the activity of genes when exposed to ursodeoxycholic acid are predicted. Groups of the placenta and parathyroid glands contained relatively few genes (nine genes each) and were characterized by a low number of relationships between members. An analysis of the specialized literature revealed the effects of taking ursodeoxycholic acid, which may be associated with the changes in gene activity, which were predicted in silico. There was no unambiguous relationship between the groups of identified in silico genes and the effects described in the literature. It was revealed that 45 genes of the entire array of genes (81 genes) are prognostic markers of oncological processes.





Similar content being viewed by others
REFERENCES
National Center for Biotechnology Information, PubChem Compound Database: CID=31401. https://pubchem.ncbi.nlm.nih.gov/compound/31401. Accessed November 15, 2018.
Minushkin, O.N., Ursodeoxycholic acid (UDCA) in clinical practice, Med. Sovet., 2010, nos. 1—2, pp. 10—11.
National Center for Biotechnology Information, PubChem Compound Database: CID=10133, https://pubchem.ncbi.nlm.nih.gov/compound/10133. Accessed November 15, 2018.
Hofmann, A.F., Pharmacology of ursodeoxycholic acid, an enterohepatic drug, Scand. J. Gastroenterol., 1994, vol. 29, no. 204, pp. 1—15. https://doi.org/10.3109/00365529409103618
Poupon, R.E., Poupon, R., Balkau, B., et al., Ursodiol for the long-term treatment of primary biliary cirrhosis, New Engl. J. Med., 1994, vol. 330, no. 19, pp. 1342—1347. https://doi.org/10.1056/NEJM199405123301903
Schiedermaier, P., Hansen, S., Asdonk, D., et al., Effects of ursodeoxycholic acid on splanchnic and systemic hemodynamics, Digestion, 2000, vol. 61, no. 2, pp. 107—112. https://doi.org/10.1159/000007742
National Center for Biotechnology Information, PubChem Compound Database: CID=9903, https://pubchem.ncbi.nlm.nih.gov/compound/9903. Accessed November 15, 2018.
Sinakos, E., Marschall, H.-U., Kowdley, K.V., et al., Bile acid changes after high-dose ursodeoxycholic acid treatment in primary sclerosing cholangitis: relation to disease progression, Hepatology, 2010, vol. 52, no. 1, pp. 197—203. https://doi.org/10.1002/hep.23631
Hofmann, A.F., The continuing importance of bile acids in liver and intestinal disease, Arch. Intern. Med., 1999, vol. 159, no. 22, pp. 2647—2658. https://doi.org/10.1001/archinte.159.22.2647
Kowdley, K.V., Ursodeoxycholic acid therapy in hepatobiliary disease, Am. J. Med., 2000, vol. 108, no. 6, pp. 481—486. https://doi.org/10.1016/s0002-9343(00)00318-1
Kotb, M.A., Ursodeoxycholic acid in neonatal hepatitis and infantile paucity of intrahepatic bile ducts: review of a historical cohort, Dig. Dis. Sci., 2009, vol. 54, no. 10, p. 2231. https://doi.org/10.1007/s10620-008-0600-8
Joo, S.S., Kang, H.C., Won, T.J., and Lee, D.I., Ursodeoxycholic acid inhibits pro-inflammatory repertoires, IL-1β and nitric oxide in rat microglia, Arch. Pharm. Res., 2003, vol. 26, no. 12, pp. 1067—1073. https://doi.org/10.1007/bf02994760
Joo, S.S., Kang, H.C., Won, T.J., and Lee, D.I., Inhibition of iNOS expression via ursodeoxycholic acid in murine microglial cell, BV-2 Cell Line, Immune Network, 2005, vol. 5, no. 1, pp. 45—49. https://doi.org/10.4110/in.2005.5.1.45
Joo, S.S., Won, T.J., and Lee, D.I., Potential role of ursodeoxycholic acid in suppression of nuclear factor kappa B in microglial cell line (BV-2), Arch. Pharm. Res., 2004, vol. 27, no. 9, p. 954. https://doi.org/10.1007/bf02975850
Biber, K., Möller, T., Boddeke, E., and Prinz, M., Central nervous system myeloid cells as drug targets: current status and translational challenges, Nat. Rev. Drug Discovery, 2016, vol. 15, no. 2, p. 110. https://doi.org/10.1038/nrd.2015.14
Wee Yong, V., Inflammation in neurological disorders: a help or a hindrance?, Neuroscientist, 2010, vol. 16, no. 4, pp. 408—420. https://doi.org/10.1177/1073858410371379
Gareth, P.J., Cecilia, R., Marcia, A.M., et al., Safety, tolerability, and cerebrospinal fluid penetration of ursodeoxycholic acid in patients with amyotrophic lateral sclerosis, Clin. Neuropharmac., 2010, vol. 33, no. 1, pp. 17—21. https://doi.org/10.1097/WNF.0b013e3181c47569
Ramalho, R.M., Viana, R.J.S., Low, W.C., et al., Bile acids and apoptosis modulation: an emerging role in experimental Alzheimer’s disease, Trends Mol. Med., 2008, vol. 14, no. 2, pp. 54—62. https://doi.org/10.1016/j.molmed.2007.12.001
Calmus, Y., Gane, P., Rouger, Ph., and Poupon, R., Hepatic expression of class I and class II major histocompatibility complex molecules in primary biliary cirrhosis: effect of ursodeoxycholic acid, Hepatology, 1990, vol. 11, no. 1, pp. 12—15. https://doi.org/10.1002/hep.1840110104
Yoshikawa, M., Tsujii, T., Matsumura, K., et al., Immunomodulatory effects of ursodeoxycholic acid on immune responses, Hepatology, 1992, vol. 16, no. 2, pp. 358—364. https://doi.org/10.1002/hep.1840160213
Quist, R.G., Ton-Nu, H-T., Lillienau, J., et al., Activation of mast cells by bile acids, Gastroenterology, 1991, vol. 101, no. 2, pp. 446—456. https://doi.org/10.1016/0016-5085(91)90024-f
Solá, S., Amaral, J.D., Aranha, M.M., et al., Modulation of hepatocyte apoptosis: cross-talk between bile acids and nuclear steroid receptors, Curr. Med. Chem., 2006, vol. 13, no. 25, pp. 3039—3051. https://doi.org/10.2174/092986706778521823
Solá, S., Amaral, J.D., Castro, R.E., et al., Nuclear translocation of UDCA by the glucocorticoid receptor is required to reduce TGFβ1–induced apoptosis in rat hepatocytes, Hepatology, 2005, vol. 42, no. 4, pp. 925—934. https://doi.org/10.1002/hep.20870
Weitzel, C., Stark, D., Kullmann, F., et al., Ursodeoxycholic acid induced activation of the glucocorticoid receptor in primary rat hepatocytes, Eur. J. Gastroenterol. Hepatol., 2005, vol. 17, no. 2, pp. 169—177. https://doi.org/10.1097/00042737-200502000-00007
Amaral, D., Solá, S., Steer, C., et al., Role of nuclear steroid receptors in apoptosis, Curr. Med. Chem., 2009, vol. 16, no. 29, pp. 3886—3902. https://doi.org/10.2174/092986709789178028
Solá, S., Ma, X., Rui, E., et al., Ursodeoxycholic acid modulates E2F-1 and p53 expression through a caspase-independent mechanism in transforming growth factor β1-induced apoptosis of rat hepatocytes, J. Biol. Chem., 2003, vol. 278, no. 49, pp. 48831—48838. https://doi.org/10.1074/jbc.M300468200
Jacquemin, E., Hermans, D., Myara, A., et al., Ursodeoxycholic acid therapy in pediatric patients with progressive familial intrahepatic cholestasis, Hepatology, 1997, vol. 25, no. 3, pp. 519—523. https://doi.org/10.1002/hep.510250303
Albensi, B.C. and Mattson, M.P., Evidence for the involvement of TNF and NFκB in hippocampal synaptic plasticity, Synapse, 2000, vol. 35, no. 2, pp. 151—159. https://doi.org/10.1002/(SICI)1098-2396(200002)35:2<151::AID-SYN8>3.0.CO;2-P
Gilmore, T.D., Introduction to NF-κB: players, pathways, perspectives, Oncogene, 2006, vol. 25, no. 51, p. 6680. https://doi.org/10.1038/sj.onc.1209954
Perkins, N.D., Integrating cell-signalling pathways with NF-κB and IKK function, Nat. Rev. Mol. Cell Biol., 2007, vol. 8, no. 1, p. 49. https://doi.org/10.1038/nrm2083
Miura, T., Ouchida, R., Yoshikawa, N., et al., Functional modulation of the glucocorticoid receptor and suppression of NF-κB-dependent transcription by ursodeoxycholic acid, J. Biol. Chem., 2001, vol. 276, no. 50, pp. 47371—47378. https://doi.org/10.1074/jbc.M107098200
Schuster, A., Schilling, T., Laurenzi, V., et al., ΔNp73β is oncogenic in hepatocellular carcinoma by blocking apoptosis signaling via death receptors and mitochondria, Cell Cycle, 2010, vol. 9, no. 13, pp. 2629—2639. https://doi.org/10.4161/cc.9.13.12110
Masferrer, J.L. and Seibert, K., Regulation of prostaglandin synthesis by glucocorticoids, Receptor, 1994, vol. 4, no. 1, pp. 25—30.
Goppelt-Struebe, M., Molecular mechanisms involved in the regulation of prostaglandin biosynthesis by glucocorticoids, Biochem. Pharm., 1997, vol. 53, no. 10, pp. 1389—1395. https://doi.org/10.1038/sj.ki.5002058
Ikegami, T., Matsuzaki, Y., Fukushima, S., et al., Suppressive effect of ursodeoxycholic acid on type IIA phospholipase A2 expression in HepG2 cells, Hepatology, 2005, vol. 41, no. 4, pp. 896—905. https://doi.org/10.1002/hep.20630
Mitsuyoshi, H., Nakashima, T., Inaba, K., et al., Ursodeoxycholic acid enhances glucocorticoid-induced tyrosine aminotransferase-gene expression in cultured rat hepatocytes, Bioch. Biophys. Res. Commun., 1997, vol. 240, no. 3, pp. 732—736. https://doi.org/10.1006/bbrc.1997.7733
Luisi, B.F., Xu, W.X., Otwinowski, Z., et al., Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA, Nature, 1991, vol. 352, no. 6335, P. 497. https://doi.org/10.1038/352497a0
Savory, J.G.A., Préfontaine, G.G., Lamprecht, C., et al., Glucocorticoid receptor homodimers and glucocorticoid-mineralocorticoid receptor heterodimers form in the cytoplasm through alternative dimerization interfaces, Mol. Cell. Biol., 2001, vol. 21, no. 3, pp. 781—793. https://doi.org/10.1128/MCB.21.3.781-793.2001
Solá, S., Castro, R.E., Kren, B.T., et al., Modulation of nuclear steroid receptors by ursodeoxycholic acid inhibits TGF-β1-induced E2F-1/p53-mediated apoptosis of rat hepatocytes, Biochemistry, 2004, vol. 43, no. 26, pp. 8429—8438. https://doi.org/10.1021/bi049781x
Shi, Q.Y., Kong, B.H., Ma, K.D., et al., Effects of ursodeoxycholic acid on the liver plasma membrane fluidity, hepatic glutathione concentration, hepatic estrogen receptors and progesterone receptors in pregnant rats with ethinylestradiol and progesterone induced intrahepatic cholestasis, Zhonghua Fu Chan Ke za Zhi, 2003, vol. 38, no. 11, pp. 680—682.
Rodrigues, C.M.P., Ma, X., Linehan-Stieers, C., et al., Ursodeoxycholic acid prevents cytochrome c release in apoptosis by inhibiting mitochondrial membrane depolarization and channel formation, Cell Death Diff., 1999, vol. 6, no. 9, p. 842. https://doi.org/10.1038/sj.cdd.4400560
Nishigaki, Y., Ohnishi, H., Moriwak, H., and Muto, Y., Ursodeoxycholic acid corrects defective natural killer activity by inhibiting prostaglandin E2 production in primary biliary cirrhosis, Dig. Dis. Sci., 1996, vol. 41, no. 7, pp. 1487—1493. https://doi.org/10.1007/BF02088577
Guarino, M.P.L., Carotti, S., Morini, S., et al., Decreased number of activated macrophages in gallbladder muscle layer of cholesterol gallstone patients following ursodeoxycholic acid, Gut, 2008, vol. 57, no. 12, pp. 1740—1741. https://doi.org/10.1136/gut.2008.160333
Bachrach, W.H. and Hofmann, A.F., Ursodeoxycholic acid in the treatment of cholesterol cholelithiasis, Dig. Dis. Sci., 1982, vol. 27, no. 8, pp. 737—761. https://doi.org/10.1007/BF01393771
Zollner, G. and Trauner, M., Nuclear receptors as therapeutic targets in cholestatic liver diseases, Br. J. Pharmacol., 2009, vol. 156, no. 1, pp. 7—27. https://doi.org/10.1111/j.1476-5381.2008.00030.x
Rodrigues, C.M.P., Fan, G., Wong, P.Y., et al., Ursodeoxycholic acid may inhibit deoxycholic acid-induced apoptosis by modulating mitochondrial transmembrane potential and reactive oxygen species production, Mol. Med., 1998, vol. 4, no. 3, pp. 165—178.
Koh, H., Lee, K.H., Kim, D., et al., Inhibition of Akt and its anti-apoptotic activities by tumor necrosis factor-induced protein kinase C-related kinase 2 (PRK2) cleavage, J. Biol. Chem., 2000, vol. 275, no. 44, pp. 34451—34458. https://doi.org/10.1074/jbc.M001753200
Amaral, J.D., Castro, R.E., Solá, S., et al., p53 is a key molecular target of ursodeoxycholic acid in regulating apoptosis, J. Biol. Chem., 2007, vol. 282, no. 47, pp. 34250—34259. https://doi.org/10.1074/jbc.M704075200
Amaral, D.J., Xavier, J.M., Clifford, J.S., et al., Targeting the p53 pathway of apoptosis, Curr. Pharm. Des., 2010, vol. 16, no. 22, pp. 2493—2503. https://doi.org/10.2174/138161210791959818
Yu, J. and Zhang, L., PUMA, a potent killer with or without p53, Oncogene, 2009, vol. 27, no. S1, p. S71. https://doi.org/10.1038/onc.2009.45
Gudkov, A.V. and Komarova, E.A., The role of p53 in determining sensitivity to radiotherapy, Nat. Rev. Cancer, 2003, vol. 3, no. 2, p. 117. https://doi.org/10.1016/j.ijrobp.2004.03.005
Joerger, A.C. and Fersht, A.R., Structural biology of the tumor suppressor p53, Annu. Rev. Biochem., 2008, vol. 77, pp. 557—582. https://doi.org/10.1146/annurev.biochem.77.060806.091238
Park, I.H., Kim, M.K., and Kim, S.U., Ursodeoxycholic acid prevents apoptosis of mouse sensory neurons induced by cisplatin by reducing p53 accumulation, Biochem. Biophys. Res. Commun., 2008, vol. 377, no. 4, pp. 1025—1030. https://doi.org/10.1016/j.bbrc.2008.06.014
Ji, W.J., Qu, Q., Jin, Y., et al., Ursodeoxycholic acid inhibits hepatocyte-like cell apoptosis by down-regulating the expressions of Bax and Caspase-3, Zhonghua Yi Xue Za Zhi, 2009, vol. 89, no. 42, pp. 2997—3001.
Hess, J., Angel, P., and Schorpp-Kistner, M., AP-1 subunits: quarrel and harmony among siblings, J. Cell Sci., 2004, vol. 117, no. 25, pp. 5965—5973. https://doi.org/10.1242/jcs.01589
Im, E. and Martinez, J.D., Ursodeoxycholic acid (UDCA) can inhibit deoxycholic acid (DCA)-induced apoptosis via modulation of EGFR/Raf-1/ERK signaling in human colon cancer cells, J. Nutr., 2004, vol. 134, no. 2, pp. 483—486. https://doi.org/10.1093/jn/134.2.483
Burnat, G., Majka, J., and Konturek, P.C., Bile acids are multifunctional modulators of the Barrett’s carcinogenesis, J. Physiol. Pharmac., 2010, vol. 61, no. 2, p. 185.
Martinez-Diez, M.C., Serrano, M.A., Monte, M.J., and Marin, J.J.G., Comparison of the effects of bile acids on cell viability and DNA synthesis by rat hepatocytes in primary culture, Biochim. Biophys. Acta,Mol. Basis Dis., 2000, vol. 1500, no. 2, pp. 153—160. https://doi.org/10.1016/s0925-4439(99)00099-x
Ashley, A.P., Akare, S., Wenqing, Qi., et al., Resistance to ursodeoxycholic acid-induced growth arrest can also result in resistance to deoxycholic acid-induced apoptosis and increased tumorgenicity, BMC Cancer, 2006, vol. 6, no. 1, p. 219. https://doi.org/10.1186/1471-2407-6-219
Garewal, H., Bernstein, H., Bernstein, C., et al., Reduced bile acid-induced apoptosis in “normal” colorectal mucosa: a potential biological marker for cancer risk, Cancer Res., 1996, vol. 56, no. 7, pp. 1480—1483.
Castro, R.E., Amaral, J.D., Solá, S., et al., Differential regulation of cyclin D1 and cell death by bile acids in primary rat hepatocytes, Am. J. Physiol.: Gastrointest. Liver Physiol., 2007, vol. 293, no. 1, pp. G327—G334. https://doi.org/10.1152/ajpgi.00093.2007
He, Y., Liu, Z., Qiao, C., et al., Expression and significance of Wnt signaling components and their target genes in breast carcinoma, Mol. Med. Rep., 2014, vol. 9, no. 1, pp. 137—143. https://doi.org/10.3892/mmr.2013.1774
Zasadkevich, Yu.M. and Sazonov, S.V., The role of E-cadherin cell adhesion molecule in human ontogenesis in norm and pathology, Morfologiya, 2014, vol. 146, no. 5, pp. 78—82.
Akare, S., Jean-Louis, S., Chen, W., et al., Ursodeoxycholic acid modulates histone acetylation and induces differentiation and senescence, Int. J. Cancer, 2006, vol. 119, no. 12, pp. 2958—2969. https://doi.org/10.1002/ijc.22231
Huang, J., Plass, C., and Gerhauser, C., Cancer chemoprevention by targeting the epigenome, Curr. Drug Targets, 2011, vol. 12, no. 13, pp. 1925—1956. https://doi.org/10.2174/138945011798184155
Oike, T., Ogiwara, H., Torikai, K., et al., Garcinol, a histone acetyltransferase inhibitor, radiosensitizes cancer cells by inhibiting non-homologous end joining, Int. J. Radiation Oncol., Biol., Physics, 2012, vol. 84, no. 3, pp. 815—821. https://doi.org/10.1016/j.ijrobp.2012.01.017
Paumgartner, G. and Beuers, U., Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited, Hepatology, 2002, vol. 36, no. 3, pp. 525—531. https://doi.org/10.1053/jhep.2002.36088
Lazaridis, K.N., Gores, G.J., and Lindor, K.D., Ursodeoxycholic acid ‘mechanisms of action and clinical use in hepatobiliary disorders’, J. Hepatol., 2001, vol. 35, no. 1, pp. 134—146. https://doi.org/10.1016/s0168-8278(01)00092-7
Rodrigues, C.M., Fan, G., Ma, X., et al., A novel role for ursodeoxycholic acid in inhibiting apoptosis by modulating mitochondrial membrane perturbation, J. Clin. Invest., 1998, vol. 101, no. 12, pp. 2790—2799. https://doi.org/10.1172/JCI1325
Abate, N., Carubbi, F., Bozzoli, M., et al., Effect of chenodeoxycholic acid and ursodeoxycholic acid administration on acyl-CoA: cholesterol acyltransferase activity in human liver, Ital. J. Gastroenterol., 1994, vol. 26, no. 6, pp. 287—293.
Smith, L.L., Another cholesterol hypothesis: cholesterol as antioxidant, Free Radical Biol. Med., 1991, vol. 11, no. 1, pp. 47—61. https://doi.org/10.1016/0891-5849(91)90187-8
DuSell, C.D., Nelson, E.R., Wang, X., et al., The endogenous selective estrogen receptor modulator 27-hydroxycholesterol is a negative regulator of bone homeostasis, Endocrinology, 2010, vol. 151, no. 8, pp. 3675—3685. https://doi.org/10.1210/en.2010-0080
Galluzzi, L., Morselli, E., Kepp, O., et al., Mitochondrial gateways to cancer, Mol. Aspects Med., 2010, vol. 31, no. 1, pp. 1—20. https://doi.org/10.1016/j.mam.2009.08.002
Bouscarel, B., Gettys, T.W., Fromm, H., and Dubner, H., Ursodeoxycholic acid inhibits glucagon-induced cAMP formation in hamster hepatocytes: a role for PKC, Am. J. Physiol.: Gastrointest. Liver Physiol., 1995, vol. 268, no. 2, pp. G300—G310. https://doi.org/10.1152/ajpgi.1995.268.2.G300
Bouscarel, B., Matsuzaki, Y., Le, M., et al., Changes in G protein expression account for impaired modulation of hepatic cAMP formation after BDL, Am. J. Physiol.: Gastrointest. Liver Physiol., 1998, vol. 274, no. 6, pp. G1151—G1159. https://doi.org/10.1152/ajpgi.1998.274.6.G1151
Peterson, T.C., Slysz, G., and Isbrucker, R., The inhibitory effect of ursodeoxycholic acid and pentoxifylline on platelet derived growth factor-stimulated proliferation is distinct from an effect by cyclic AMP, Immunopharmacology, 1998, vol. 39, no. 3, pp. 181—191. https://doi.org/10.1016/s0162-3109(98)00021-6
Higuchi, H., Grambihler, A., Canbay, A., et al., Bile acids up-regulate death receptor 5/TRAIL-receptor 2 expression via a c-Jun N-terminal kinase-dependent pathway involving Sp1, J. Biol. Chem., 2004, vol. 279, no. 1, pp. 51—60. https://doi.org/10.1074/jbc.M309476200
Andersson, Y., Juell, S., and Fodstad, O., Downregulation of the antiapoptotic MCL-1 protein and apoptosis in MA-11 breast cancer cells induced by an anti-epidermal growth factor receptor–Pseudomonas exotoxin a immunotoxin, Int. J. Cancer, 2004, vol. 112, no. 3, pp. 475—483. https://doi.org/10.1002/ijc.20371
Rolo, A.P., Palmeira, C.M., Holy, J.M., and Wallace, K.B., Role of mitochondrial dysfunction in combined bile acid-induced cytotoxicity: the switch between apoptosis and necrosis, Toxicol. Sci., 2004, vol. 79, no. 1, pp. 196—204. https://doi.org/10.1093/toxsci/kfh078
Paolini, M., Pozzetti, L., Montagnani, M., et al., Ursodeoxycholic acid (UDCA) prevents DCA effects on male mouse liver via up-regulation of CXP and preservation of BSEP activities, Hepatology, 2002, vol. 36, no. 2, pp. 305—314. https://doi.org/10.1053/jhep.2002.34939
Pemberton, P.W., Aboutwerat, A., Smith, A., and Warnes, T.W., Ursodeoxycholic acid in primary biliary cirrhosis improves glutathione status but fails to reduce lipid peroxidation, Redox Rep., 2006, vol. 11, no. 3, pp. 117—123. https://doi.org/10.1179/135100006X116600
Zollner, G., Wagner, M., Moustafa, T., et al., Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-α/β in the adaptive response to bile acids, Am. J. Physiol.: Gastrointest. Liver Physiol., 2006, vol. 290, no. 5, pp. G923—G932. https://doi.org/10.1152/ajpgi.00490.2005
Poroikov, V.V. and Filimonov, D.A., Komp’yuternyi prognoz biologicheskoi aktivnosti khimicheskikh soedinenii kak osnova dlya poiska i optimizatsii bazovykh struktur novykh lekarstv (Computer Prediction of the Biological Activity of Chemical Compounds as a Basis for the Search and Optimization of the Basic Structures of New Drugs), Moscow: : Iridium-Press, 2001, pp. 123—125.
Lagunin, A., Ivanov, S., Rudik, A., et al., DIGEP-Pred: web service for in silico prediction of drug-induced gene expression profiles based on structural formula, Bioinformatics, 2013, vol. 29, no. 16, pp. 2062—2063. https://doi.org/10.1093/BIOINFORMATICS/BTT322
Warde-Farley, D., Donaldson, S.L., Comes, O., et al., The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function, Nucleic Acids Res., 2010, vol. 38, suppl. 2, pp. W214—W220. https://doi.org/10.1093/NAR/GKQ537
Diss, G. and Lehner, B., The genetic landscape of a physical interaction, eLife, 2018, vol. 7, p. e32472. https://doi.org/10.7554/eLife.32472
Boucher, B. and Jenna, S., Genetic interaction networks: better understand to better predict, Front. Genet., 2013, vol. 4, p. 290. https://doi.org/10.3389/fgene.2013.00290
Uhlen, M., Zhang, C., Lee, S., et al., A pathology atlas of the human cancer transcriptome, Science, 2017, vol. 357, no. 6352, p. eaan2507. https://doi.org/10.1126/science.aan2507
Thul, P.J., Åkesson, L., Wiking, M., et al., A subcellular map of the human proteome, Science, 2017, vol. 356, no. 6340. p. eaal3321. https://doi.org/10.1126/science.aal3321
Uhlén, M., Fagerberg, L., Hallström, B.M., et al., Tissue-based map of the human proteome, Science, 2015, vol. 347, no. 6220, p. 1260419. https://doi.org/10.1126/science.1260419
Zhang, P., Cao, H.Y., Bai, L.L., et al., The high expression of TC1 (C8orf4) was correlated with the expression of β-catenin and cyclin D1 and the progression of squamous cell carcinomas of the tongue, Tumor Biol., 2015, vol. 36, no. 9, pp. 7061—7067. https://doi.org/10.1007/s13277-015-3423-1
Demidenko, G.V., Kolchanov, N.A., Likhoshvai, V.A., et al., Mathematical modeling of regular contours of gene networks, Zh. Vychisl. Mat. Mat. Fiz., 2004, vol. 44, no. 12, pp. 2276—2295.
Kolchanov, N.A., Ananko, E.A., Likhoshvai, V.A., et al., Gene networks description and modeling in the GeneNet system, in Gene Regulation and Metabolism: Postgenomic Computational Approaches, Collado-Vides, J. and Hofestadt, R., Eds., MIT Press, 2002, pp. 149—179.
Kwon, Y.I., Yeon, J.D., Oh, S.M., and Chung, K.H., Protective effects of ursodeoxycholic acid against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced testicular damage in mice, Toxicol. Appl. Pharmacol., 2004, vol. 194, no. 3, pp. 239—247. https://doi.org/10.1016/j.taap.2003.09.024
Saad, R.A. and Mahmoud, Y.I., Ursodeoxycholic acid alleviates cholestasis-induced histophysiological alterations in the male reproductive system of bile duct-ligated rats, Reprod. Toxicol., 2014, vol. 50, pp. 87—97. https://doi.org/10.1016/j.reprotox.2014.10.011
Mahmoud, Y.I., Testicular immunohistochemical and ultrastructural changes associated with chronic cholestasis in rats: effect of ursodeoxycholic acid, Life Sci., 2015, vol. 136, pp. 52—59. https://doi.org/10.1016/j.lfs.2015.05.027
Zhang, X., Chen, S., Yoo, S., et al., Mutation in nuclear pore component NUP155 leads to atrial fibrillation and early sudden cardiac death, Cell, 2008, vol. 135. №. 6, pp. 1017—1027. https://doi.org/10.1016/j.cell.2008.10.022
Balagoni, H., Devani, K., Phemister, J., and Young, M.F., Ursodiol-associated paroxysmal atrial fibrillation, Am. J. Ther., 2017, vol. 24, no. 5, pp. e612—e613. https://doi.org/10.1097/MJT.0000000000000570
Rainer, P.P., Primessnig, U., Harenkamp, S., et al., Bile acids induce arrhythmias in human atrial myocardium—implications for altered serum bile acid composition in patients with atrial fibrillation, Heart, 2013, vol. 99, no. 22, pp. 1685—1692. https://doi.org/10.1136/heartjnl-2013-304163
Chen, T., Zhou, L., Zhou, Y., et al., HJURP promotes epithelial-to-mesenchymal transition via upregulating SPHK1 in hepatocellular carcinoma, Int. J. Biol. Sci., 2019, vol. 15, no. 6, p. 1139. https://doi.org/10.7150/ijbs.30904
Kuiper, E.M.M., Hansen, B.E., Adang, R.P.R., et al., Relatively high risk for hepatocellular carcinoma in patients with primary biliary cirrhosis not responding to ursodeoxycholic acid, Eur. J. Gastroenterol. Hepatology, 2010, vol. 22, no. 12, pp. 1495—1502. https://doi.org/10.1097/MEG.0b013e32834059e7
Rudolph, G., Gotthardt, D.N., Kloeters-Plachky, P., et al., In PSC with colitis treated with UDCA, most colonic carcinomas develop in the first years after the start of treatment, Dig. Dis. Sci., 2011, vol. 56, no. 12, pp. 3624—3630. https://doi.org/10.1007/s10620-011-1763-2
Nye, J., Sturgill, D., Athwal, R., and Dalal, Y., HJURP antagonizes CENP-A mislocalization driven by the H3.3 chaperones HIRA and DAXX, PLoS One, 2018, vol. 13, no. 10, p. e0205948. https://doi.org/10.1371/journal.pone.0205948
Shimamoto, A., Nishikawa, K., Kitao, S., and Furuichi, Y., Human RecQ5β, a large isomer of RecQ5 DNA helicase, localizes in the nucleoplasm and interacts with topoisomerases 3α and 3β, Nucleic Acids Res., 2000, vol. 28, no. 7, pp. 1647—1655. https://doi.org/10.1093/nar/28.7.1647
Abdelrahman, H.A., John, A., Ali, B.R., and Al-Gazali, L., Further delineation of the microcephaly-micromelia syndrome associated with loss-of-function variants in DONSON, Mol. Syndromol., 2019, vol. 10, no. 3, pp. 171—176. https://doi.org/10.1159/000497337
Fimognari, C., Nüsse, M., Cesari, R., et al., Micronuclei induction, cell cycle delay and apoptosis as markers of cellular stress caused by ursodeoxycholic acid in human lymphocytes, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2001, vol. 495, nos. 1—2, pp. 1—9. https://doi.org/10.1016/s1383-5718(01)00197-8
García, E., Marcos-Gutiérrez, C., del Mar Lorente, M, et al., RYBP, a new repressor protein that interacts with components of the mammalian Polycomb complex, and with the transcription factor YY1, EMBO J., 1999, vol. 18, no. 12, pp. 3404—3418. https://doi.org/10.1093/emboj/18.12.3404
Petrov, A.M., Kasimov, M.R., and Zefirov, A.L., Brain cholesterol metabolism and its defects: linkage to neurodegenerative diseases and synaptic dysfunction, Acta Nat., 2016, vol. 8, no. 1(28), pp. 58—73.
Dietschy, J.M., Central nervous system: cholesterol turnover, brain development and neurodegeneration, Biol. Chem., 2009, vol. 390, no. 4, pp. 287—293. https://doi.org/10.1515/BC.2009.035
Wassif, C.A., Zhu, P., Kratz, L., et al., Biochemical, phenotypic and neurophysiological characterization of a genetic mouse model of RSH/Smith—Lemli—Opitz syndrome, Hum. Mol. Genet., 2001, vol. 10, no. 6, pp. 555—564. https://doi.org/10.1093/hmg/10.6.555
Sparks, S.E., Wassif, C.A., Goodwin, H., et al., Decreased cerebral spinal fluid neurotransmitter levels in Smith—Lemli—Opitz syndrome, J. Inherited Metab. Dis., 2014, vol. 37, no. 3, pp. 415—420. https://doi.org/10.1007/s10545-013-9672-5
Pardue, M.T. and Allen, R.S., Neuroprotective strategies for retinal disease, Prog. Retinal Eye Res., 2018, vol. 65, pp. 50—76. https://doi.org/10.1016/j.preteyeres.2018.02.002
Cortez, L.M., Campeau, J., Norman, G., et al., Bile acids reduce prion conversion, reduce neuronal loss, and prolong male survival in models of prion disease, J. Virol., 2015, vol. 89, no. 15, pp. 7660—7672. https://doi.org/10.1128/JVI.01165-15
Keene, C.D., Rodrigues, C.M.P., Eich, T., et al., Tauroursodeoxycholic acid, a bile acid, is neuroprotective in a transgenic animal model of Huntington’s disease, Proc. Natl. Acad. Sci. U.S.A., 2002, vol. 99, no. 16, pp. 10671—10676. https://doi.org/10.1073/pnas.162362299
Yanovsky, Y., Schubring, S.R., Yao, Q., et al., Waking action of ursodeoxycholic acid (UDCA) involves histamine and GABAA receptor block, PLoS One, 2012, vol. 7, no. 8, p. e42512. https://doi.org/10.1371/journal.pone.0042512
Tuem, K.B. and Atey, T.M., Neuroactive steroids: receptor interactions and responses, Front. Neurol., 2017, vol. 8, p. 442. https://doi.org/10.3389/fneur.2017.00442
Numakawa, T., Suzuki, S., Kumamaru, E., et al., BDNF function and intracellular signaling in neurons, Histol. Histopathol., 2010, vol. 25, pp. 237—258. https://doi.org/10.14670/HH-25.237
Hayashi, H., Lipid metabolism and glial lipoproteins in the central nervous system, Biol. Pharm. Bull., 2011, vol. 34, no. 4, pp. 453—461. https://doi.org/10.1248/bpb.34.453
Bron, R., Vermeren, M., Kokot, N., et al., Boundary cap cells constrain spinal motor neuron somal migration at motor exit points by a semaphorin—plexin mechanism, Neural Dev., 2007, vol. 2, no. 1, p. 21. https://doi.org/10.1186/1749-8104-2-21
Allen, N.C., Bagade, S., McQueen, M.B., et al., Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database, Nat. Genet., 2008, vol. 40, no. 7, pp. 827—834. https://doi.org/10.1038/ng.171
Wray, N.R., James, M.R., Mah, S.P., et al., Anxiety and comorbid measures associated with PLXNA2, Arch. Gen. Psychiatry, 2007, vol. 64, no. 3, pp. 318—326. https://doi.org/10.1001/archpsyc.64.3.318
Kovaleva, N.B. and Bairamova, I.Kh., Intrahepatic cholestasis of pregnancy, Ross. Zh. Gastroenterol., Gepatol.Koloproktol., 2006, vol. 16, no. 3, pp. 36—40.
Lofthouse, E.M., Torrens, C., Manousopoulou, A., et al., Ursodeoxycholic acid inhibits uptake and vasoconstrictor effects of taurocholate in human placenta, FASEB J., 2019, vol. 33, no. 7, pp. 8211—8220. https://doi.org/10.1096/fj.201900015RR
Ehnert, S., Aspera-Werz, R.H., Ruoß, M., et al., Hepatic osteodystrophy—molecular mechanisms proposed to favor its development, Int. J. Mol. Sci., 2019, vol. 20, no. 10, p. 2555. https://doi.org/10.3390/ijms20102555
Verma, A., Maxwell, J.D., Ang, L., et al., Ursodeoxycholic acid enhances fractional calcium absorption in primary biliary cirrhosis, Osteoporosis Int., 2002, vol. 13, no. 8, pp. 677—682. https://doi.org/10.1007/s001980200092
Yablokov, A.V., Fenetika: evolyutsiya, populyatsiya, priznak (Phenetics: Evolution, Population, Trait), Moscow: Nauka, 1980.
Timofeev-Resovskii, N.V. and Yablokov, A.V., Phenes, phenetics, and evolutionary biology, Priroda, 1973, no. 5, pp. 40—51.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by M. Novikova
Supplementary material
Rights and permissions
About this article
Cite this article
Neskorodov, Y.B., Mardanly, S.G. & Chuprov-Netochin, R.N. The Experience of Analyzing Biological Activity of Ursodeoxycholic Acid as Part of In Silico Prediction of the Gene Expression Profile. Russ J Genet 56, 1162–1179 (2020). https://doi.org/10.1134/S1022795420100099
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795420100099