Abstract
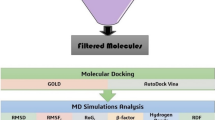
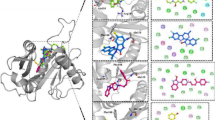
Chorismate serves as a crucial precursor for the synthesis of many aromatic compounds essential for the survival and virulence in various bacteria and protozoans. Chorismate synthase, a vital enzyme in the shikimate pathway, is responsible for the formation of chorismate from enolpyruvylshikimate-3-phosphate (EPSP). Moraxella catarrhalis is reported to be resistant to many beta-lactam antibiotics and causes chronic ailments such as otitis media, sinusitis, laryngitis, and bronchopulmonary infections. Here, we have cloned the aroC gene from Moraxella catarrhalis in pET28c and heterologously produced the chorismate synthase (~ 43 kDa) in Escherichia coli BL21(DE3) cells. We have predicted the three-dimensional structure of this enzyme and used the refined model for ligand-based virtual screening against Supernatural Database using PyRx tool that led to the identification of the top three molecules (caffeic acid, gallic acid, and o-coumaric acid). The resultant protein–ligand complex structures were subjected to 50 ns molecular dynamics (MD) simulation using GROMACS. Further, the binding energy was calculated by MM/PBSA approach using the trajectory obtained from MD simulation. The binding affinities of these compounds were validated with ITC experiments, which suggest that gallic acid has the highest binding affinity amongst these three phytochemicals. Together, these results pave the way for the use of these phytochemicals as potential anti-bacterial compounds.






Similar content being viewed by others
References
Enright MC, McKenzie H (1997) Moraxella (Branhamella) catarrhalis—clinical and molecular aspects of a rediscovered pathogen. J Med Microbiol 46:360–371. https://doi.org/10.1099/00222615-46-5-360
Hager H, Verghese A, Alvarez S, Berk SL (1987) Branhamella catarrhalis respiratory infections. Clin Infect Dis 9:1140–1149. https://doi.org/10.1093/clinids/9.6.1140
Murphy TF, Parameswaran GI (2009) Moraxella catarrhalis, a human respiratory tract pathogen. Clin Infect Dis 49:124–131. https://doi.org/10.1086/599375
Faden H, Duffy L, Wasielewski R et al (1997) Relationship between nasopharyngeal colonization and the development of otitis media in children. J Infect Dis 175:1440–1445. https://doi.org/10.1086/516477
Hendley JO, Hayden FG, Winther B (2005) Weekly point prevalence of Streptococcus pneumoniae, Hemophilus influenzae and Moraxella catarrhalis in the upper airways of normal young children: effect of respiratory illness and season. APMIS 113:213–220. https://doi.org/10.1111/j.1600-0463.2005.apm1130310.x
Karalus R, Campagnari A (2000) Moraxella catarrhalis: a review of an important human mucosal pathogen. Microbes Infect 2:547–559. https://doi.org/10.1016/S1286-4579(00)00314-2
Dev A, Tapas S, Pratap S, Kumar P (2012) Structure and function of enzymes of shikimate pathway. CurrBioinform 7:374–391. https://doi.org/10.2174/157489312803900983
Tapas S, Kumar A, Dhindwal S et al (2011) Structural analysis of chorismate synthase from Plasmodium falciparum: a novel target for antimalaria drug discovery. Int J Biol Macromol 49:767–777. https://doi.org/10.1016/j.ijbiomac.2011.07.011
Sharma A, Kumar V, Chatrath A et al (2018) In vitro metal catalyzed oxidative stress in DAH7PS: methionine modification leads to structure destabilization and induce amorphous aggregation. Int J Biol Macromol 106:1089–1106. https://doi.org/10.1016/j.ijbiomac.2017.08.105
Coggins JR, Abell C, Evans LB et al (2003) Experiences with the shikimate-pathway enzymes as targets for rational drug design. Biochem Soc Trans 31:5
Baek S-H, Rajashekara G, Splitter GA, Shapleigh JP (2004) Denitrification genes regulate brucella virulence in mice. J Bacteriol 186:6025–6031. https://doi.org/10.1128/JB.186.18.6025-6031.2004
Ahn HJ, Yoon H-J, Lee BI, Suh SW (2004) Crystal structure of chorismate synthase: a novel FMN-binding protein fold and functional insights. J Mol Biol 336:903–915. https://doi.org/10.1016/j.jmb.2003.12.072
Chook YM, Ke H, Lipscomb WN (1993) Crystal structures of the monofunctional chorismate mutase from Bacillus subtilis and its complex with a transition state analog. Proc Natl Acad Sci 90:8600–8603. https://doi.org/10.1073/pnas.90.18.8600
Quevillon-Cheruel S, Leulliot N, Meyer P et al (2004) Crystal structure of the bifunctional chorismate synthase from Saccharomyces cerevisiae. J Biol Chem 279:619–625. https://doi.org/10.1074/jbc.M310380200
Koh M, Nasertorabi F, Han GW et al (2017) Generation of an orthogonal protein-protein interface with a noncanonical amino acid. J Am Chem Soc 139:5728–5731. https://doi.org/10.1021/jacs.7b02273
Altschul SF, Gish W, Miller W et al (1990) Basic local alignment search tool. J Mol Biol 215:403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Webb B, Sali A (2016) Comparative protein structure modeling using MODELLER. CurrProtocBioinform. https://doi.org/10.1002/cpbi.3
Wiederstein M, Sippl MJ (2007) ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res 35:W407–W410. https://doi.org/10.1093/nar/gkm290
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26:283–291. https://doi.org/10.1107/S0021889892009944
Colovos C, Yeates TO (1993) Verification of protein structures: Patterns of nonbonded atomic interactions. Protein Sci 2:1511–1519. https://doi.org/10.1002/pro.5560020916
Benkert P, Künzli M, Schwede T (2009) QMEAN server for protein model quality estimation. Nucleic Acids Res 37:W510–W514. https://doi.org/10.1093/nar/gkp322
Benkert P, Tosatto SCE, Schomburg D (2008) QMEAN: a comprehensive scoring function for model quality assessment. Proteins StructFunctBioinform 71:261–277. https://doi.org/10.1002/prot.21715
Marchler-Bauer A, Lu S, Anderson JB et al (2011) CDD: a conserved domain database for the functional annotation of proteins. Nucleic Acids Res 39:D225–D229. https://doi.org/10.1093/nar/gkq1189
Sievers F, Wilm A, Dineen D et al (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. https://doi.org/10.1038/msb.2011.75
Gouet P, Courcelle E, Stuart D, Metoz F (1999) ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics 15:305–308. https://doi.org/10.1093/bioinformatics/15.4.305
Dallakyan S, Olson AJ (2015) Small-molecule library screening by docking with PyRx. In: Hempel JE, Williams CH, Hong CC (eds) Chemical biology. Springer, New York, pp 243–250
Dunkel M (2006) SuperNatural: a searchable database of available natural compounds. Nucleic Acids Res 34:D678–D683. https://doi.org/10.1093/nar/gkj132
Abraham MJ, Murtola T, Schulz R et al (2015) GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2:19–25. https://doi.org/10.1016/j.softx.2015.06.001
van Aalten DMF, Bywater R, Findlay JBC et al (1996) PRODRG, a program for generating molecular topologies and unique molecular descriptors from coordinates of small molecules. J Comput Aided Mol Des 10:255–262. https://doi.org/10.1007/BF00355047
Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 7:42717. https://doi.org/10.1038/srep42717
Fernandes CL, Santos DS, Basso LA, de Souza ON (2005) Structure prediction and docking studies of chorismate synthase from mycobacterium tuberculosis. In: Setubal JC, Verjovski-Almeida S (eds) Advances in bioinformatics and computational biology. Springer, Berlin, pp 118–127
Singh S, Qureshi IA (2020) Identification and validation of novel inhibitor against chorismate synthase of toxoplasma gondii. Social Science Research Network, Rochester
Rodrigues-Vendramini FAV, Marschalk C, Toplak M et al (2018) Promising new antifungal treatment targeting chorismate synthase from Paracoccidioides brasiliensis. Antimicrob Agents Chemother 63:e01097-18. https://doi.org/10.1128/AAC.01097-18
Arcuri HA, Palma MS (2011) Understanding the structure, activity and inhibition of chorismate synthase from Mycobacterium tuberculosis. Curr Med Chem 18:1311–1317. https://doi.org/10.2174/092986711795029528
Thomas MG, Lawson C, Allanson NM et al (2003) A series of 2(Z)-2-benzylidene-6,7-dihydroxybenzofuran-3[2H]-ones as inhibitors of chorismate synthase. Bioorg Med Chem Lett 13:423–426. https://doi.org/10.1016/S0960-894X(02)00957-5
Pitchandi P, Hopper W, Rao R (2013) Comprehensive database of chorismate synthase enzyme from shikimate pathway in pathogenic bacteria. BMC Pharmacol Toxicol 14:29. https://doi.org/10.1186/2050-6511-14-29
Newman DJ, Cragg GM (2020) Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod 83:770–803. https://doi.org/10.1021/acs.jnatprod.9b01285
Li JW-H, Vederas JC (2009) Drug discovery and natural products: end of an era or an endless frontier? Science 325:161–165. https://doi.org/10.1126/science.1168243
Wang H (2004) Determination of rosmarinic acid and caffeic acid in aromatic herbs by HPLC. Food Chem 87:307–311. https://doi.org/10.1016/j.foodchem.2003.12.029
Shan B, Cai YZ, Sun M, Corke H (2005) Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J Agric Food Chem 53:7749–7759. https://doi.org/10.1021/jf051513y
Al-Farsi M, Alasalvar C, Morris A et al (2005) Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J Agric Food Chem 53:7592–7599. https://doi.org/10.1021/jf050579q
Zheng W, Wang SY (2003) Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J Agric Food Chem 51:502–509. https://doi.org/10.1021/jf020728u
Hashmi MA, Khan A, Hanif M et al (2015) Traditional uses, phytochemistry, and pharmacology of Olea europaea (Olive). Evid Based Complement Alternat Med 2015:541591. https://doi.org/10.1155/2015/541591
Magnani C, Isaac VLB, Correa MA, Salgado HRN (2014) Caffeic acid: a review of its potential use in medications and cosmetics. Anal Methods 6:3203–3210. https://doi.org/10.1039/C3AY41807C
Espíndola KMM, Ferreira RG, Narvaez LEM et al (2019) Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front Oncol 9:541. https://doi.org/10.3389/fonc.2019.00541
Alves MJ, Ferreira ICFR, Froufe HJC et al (2013) Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J Appl Microbiol 115:346–357. https://doi.org/10.1111/jam.12196
Choubey S, Varughese LR, Kumar V, Beniwal V (2015) Medicinal importance of gallic acid and its ester derivatives: a patent review. Pharm Pat Anal 4:305–315. https://doi.org/10.4155/ppa.15.14
Acknowledgments
NN thanks to CSIR, MS, thanks to MHRD; JKM, thanks to DBT for providing financial assistance. The authors also thank the Macromolecular Crystallographic Facility (MCU) at IIC, Indian Institute of Technology Roorkee, for using the facility to carry out experiments.
Funding
This work was supported by a grant from the Science and Engineering Research Board (SERB), India (Project No. CRG/2018/001022).
Author information
Authors and Affiliations
Contributions
NN, MS, and PK designed the study. NN, MS, JKM performed the cloning, expression, purification, and biophysical experiments. MS, JKM performed in-silico studies and analysis. NN, MS, JKM, and PK wrote the manuscript. All the authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest/competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Neetu, N., Sharma, M., Mahto, J.K. et al. Biophysical and In-Silico Studies of Phytochemicals Targeting Chorismate Synthase from Drug-Resistant Moraxella Catarrhalis. Protein J 39, 449–460 (2020). https://doi.org/10.1007/s10930-020-09923-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-020-09923-y