Abstract
Composite plasmonic structures consisting of gold nanoparticles and carbon nanodots (CDs) in mesoporous silica particles have been synthesized. It has been established that the visible photoluminescence of CDs in these structures is three times more intense than that in analogous structures free of gold nanoparticles.
Similar content being viewed by others
Carbon nanodots (C-dots, CDs) synthesized by inexpensive technological methods possessing a number of unique physical and chemical properties and offering promising applications have been extensively studied in the past decade [1–3]. In comparison to the well-known semiconductor quantum dots (QDs), CDs exhibit a number of advantages, including biocompatibility, low toxicity, good aqueous solubility, chemical inertness, and photostability [1–3]. The interest of researchers has been mostly devoted to the luminescent properties of CDs, which can provide a basis for numerous potential biomedical applications [1–3], in particular, as markers and biosensors.
The enhancement of the intensity of photoluminescence (PL) from CDs that is necessary for optical and biomedical applications can be achieved due to the interaction of CDs with metal plasmonic nanostructures situated at certain distance [4]. In particular, it has been reported [5] that CD solution applied onto a substrate bearing silver nanoparticles (AgNPs) exhibited 1.5- to 10-fold increased PL intensity (depending on the excitation conditions) and improved photostability as compared to the same solution on a glass substrate without metal nanoparticles. Another approach to obtaining spatially separated CDs and AgNPs was implemented [6] by introducing these particles into a reaction mixture used for the synthesis of spherical particles of mesoporous silica (mSiO2). The PL intensity of CDs in the composite particles increased by about three times as compared to that in the initial solution [6].
It should be noted that mSiO2 particles offer a promising template for biomedical applications [7, 8] due to their high adsorption capacity, possibility of additional modification, biocompatibility, and a number of other functional properties. For example, multifunctional hybrid particles with a structure of the composite core–shell (mSiO2/Gd2O3 : Eu3+) type on this basis proved to be capable of simultaneously performing therapeutic (neutron-capture therapy with targeted delivery of drugs) and diagnostic functions (as a contrast agent in magnetic-resonance tomography and luminescent marker) [9]. Metal nanoparticles and CDs in multifunctional mSiO2-based particles frequently play a determining role in biomedical applications [10, 11]. For example, mesoporous mSiO2 containing gold nanoparticles (AuNPs) in the pores were recently successfully employed for combined immunotherapy and photothermal therapy of cancer [10]. Luminescent hybrid particles comprising an mSiO2 core covered with ATP-aptamer conjugated by π-bonds with graphene CDs have been used for monitoring of the aptamer-controlled release of anticancer drug (doxorubicin) from mSiO2 pores in HeLa cells [11].
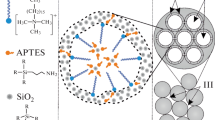
In the present work, we have used a template method for the synthesis of composite structures comprising monodisperse mSiO2 particles containing AuNPs and CDs possessing bright PL in the visible spectral range, which is additionally enhanced due to the interaction with AuNPs.
The monodisperse mSiO2 particles used as a template for the synthesis of composite structures were prepared by hydrolysis of tetraethoxysilane in alcohol–water–ammonia medium containing a structure-forming surfactant agent [12]. These particles contained a system of closely packed monodisperse cylindrical pores with an average diameter of 3.1 ± 0.2 nm. The pore volume fraction was about 50% of the particle volume. The mSiO2 particles had an average diameter of 450 ± 20 nm.
Gold nanoparticles in the mSiO2 template were formed in two steps. At the first stage, an aqueous HAuCl4 solution was introduced into template pores under the action of capillary forces followed by drying under normal conditions. At the second stage, mSiO2 particles with a precursor in the pores were annealed in hydrogen flow at 200°C until complete conversion of gold chlorides into metallic Au. The volume fraction of elemental gold in mSiO2/Au composite particles amounted to 2 vol %.
The synthesis of CDs in pores of mSiO2 and mSiO2/Au particles was performed via thermal decomposition of a carbon-containing precursor [13, 14]. The initial particles were impregnated by Rhodamine 6G (R6G) dye solution followed by heat treatment in air at 280oC. The content of CDs in mSiO2/СD and mSiO2/Au/СD composite particles was the same and amounted to ~2 vol %. The values of the specific surface and pore volume in mSiO2/Au/СD composite particles according to the data of adsorption structural analysis were 420 m2/g and 0.3 cm3/g, respectively.
The optical absorption spectra of the obtained composite structures in a 300–800 nm wavelength range I (Fig. 1) were measured using a Shimadzu UV 3600 Plus spectrophotometer with integrating sphere. Sample suspensions with the same concentration of particles (0.05 vol %) were placed in quartz cells. The PL spectra (Fig. 2) of the same suspensions were measured under excitation by He–Cd and Nd–YAG lasers at 325- and 532-nm wavelengths, respectively. The measurements were performed at room temperature and recorded with the aid of a computer-controlled MDR-23 monochromator with photomultiplier detector operating in the photon count mode.
The absorption spectrum of mSiO2/СD particles (Fig. 1, curve 1) displays a band with a maximum at 519 nm and full width at half maximum (FWHM) of ~69 nm, which is attributed to the absorption of probing radiation by fragments of polyaromatic xanthene groups of R6G precursor incorporated into graphene layers forming the nanodots [14]. The absorption band observed in this spectral region in the spectrum of mSiO2/Au/СD particles (Fig. 1, curve 2) has a greater width (FWHM ~ 77 nm), an about 1.7-times-higher intensity, and its maximum is shifted to longer wavelengths (λmax ~ 524 nm), which is related to absorption by xanthene fragments of CDs and the plasmonic absorption band of AuNPs [15]. The optical absorption in the region of 300–450 nm observed in the spectra of composite particles of both types is probably related to the absorption by CDs [13, 14] and AuNPs [15]. For the comparison, Fig. 1 also shows the spectrum of unfilled mSiO2 particles with an average diameter of 450 nm (curve 3) in which the structureless optical absorption is small.
The PL spectra (Fig. 2) of suspensions with the same concentration (0.05 vol %) of mSiO2/CD and mSiO2/Au/CD composite particles containing equal amounts (2 vol %) of CDs exhibit emission bands related to the luminescence of CDs synthesized from R6G [15]. Both the positions of the maxima and shapes of PL bands of the composite particles with and without AuNPs under identical excitation are close. For the excitation by radiation with different wavelengths (325 and 532 nm), the positions of PL maxima differ and correspond to 538 and 562 nm, respectively. An observed shift of the PL peak position toward longer wavelengths with increasing excitation wavelength is characteristic of CDs [16]. At both excitation wavelengths, the intensity of PL from particles containing AuNP/CD plasmonic nanostructures is about 2.8 times as large as that from gold-free composite particles.
Thus, we have used a template method for the synthesis of plasmonic structures consisting of CDs and AuNPs, which exhibit bright PL in the visible spectral range. The role of the template was played by monodisperse spherical mesoporous silica particles. Rhodamine 6G was selected as precursor for the synthesis of CDs because polyaromatic fragments formed upon its thermodestruction exhibit luminescence in the spectral region of the plasmonic absorption band of AuNPs, which is retained when these fragments are incorporated into graphene layers of CDs. Composite mSiO2/Au/CD particles readily dissolve in water to form stable suspension and possess large specific surface and pore volume (420 m2/g and 0.3 cm3/g, respectively). It is established that the intensity of PL from mSiO2/Au/CD particles in the obtained plasmonic structures is about three times as large as that in analogous structures free of gold nanoparticles.
REFERENCES
S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed. Engl. 49, 6726 (2010).
O. Kargbo, Y. Jin, and S.-Y. Ding, Curr. Anal. Chem. 11, 4 (2015).
F. Yan, Z. Sun, H. Zhang, X. Sun, Y. Jiang, and Z. Bai, Microchim. Acta 186, 583 (2019).
S. T. Kochuveedu and D. H. Kim, Nanoscale 6, 4966 (2014).
Y. Zhang, H. Goncalves, J. C. G. E. Silva, and C. D. Geddes, Chem. Commun. 47, 5313 (2011).
Y. Liu, C. Liu, Z. Zhang, W. Yang, and S. Nie, J. Mater. Chem. C 3, 2881 (2015).
Q. He and J. Shi, Adv. Mater. 26, 391 (2014).
Y. Shi, M. L. Miller, and A. J. Di Pasqua, Comm. Inorg. Chem. 36, 61 (2016).
D. A. Eurov, D. A. Kurdyukov, D. A. Kirilenko, J. A. Kukushkina, A. V. Nashchekin, A. N. Smirnov, and V. G. Golubev, J. Nanopart. Res. 17, 82 (2015).
C. Ong, B. G. Cha, and J. Kim, ACS Appl. Bio Mater. 2, 3630 (2019).
F. Zheng, P. Zhang, Y. Xi, J. Chen, L. Li, and J.-J. Zhu, Anal. Chem. 87, 11739 (2015).
D. A. Kurdyukov, D. A. Eurov, D. A. Kirilenko, J. A. Kukushkina, V. V. Sokolov, M. A. Yagovkina, and V. G. Golubev, Microporous Mesoporous Mater. 223, 225 (2016).
D. A. Kurdyukov, D. A. Eurov, E. Yu. Stovpyaga, D. A. Kirilenko, S. V. Konyakhin, A. V. Shvidchenko, and V. G. Golubev, Phys. Solid State 58, 2545 (2016).
D. A. Eurov, D. A. Kurdyukov, A. V. Medvedev, and V. G. Golubev, Tech. Phys. Lett. 45, 940 (2019).
V. Amendola, R. Pilot, M. Fracconi, O. M. Maragò, and M. A. Iatì, J. Phys.: Condens. Matter 29, 37 (2017).
D. K. Nelson, B. S. Razbirin, A. N. Starukhin, D. A. Eurov, D. A. Kurdyukov, E. Y. Stovpiaga, and V. G. Golubev, Opt. Mater. 59, 28 (2016).
ACKNOWLEDGMENTS
The authors are grateful to L.V. Sharonova for measuring the absorption spectra of synthesized particles.
Funding
This work was supported in part by the Russian Foundation for Basic Research, project no. 18-29-19122_mk.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by P. Pozdeev
Rights and permissions
About this article
Cite this article
Kurdyukov, D.A., Eurov, D.A., Medvedev, A.V. et al. Luminescent Plasmonic Structures Based on Gold Nanoparticles and Carbon Nanodots in Mesoporous Silica Particles. Tech. Phys. Lett. 46, 928–930 (2020). https://doi.org/10.1134/S1063785020090229
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063785020090229