Abstract
Main conclusion
This study highlights the potential link between high light-induced canopy-level photosynthesis and mesophyll cell K+, Cl−, Ca2+, and H+ homeostasis in tomato.
Abstract
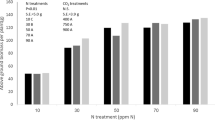
Light is a primary energy source for photosynthesis and a vital regulator of mineral nutrient uptake and distribution in plants. Plants need to optimize photosynthesis and nutrient balance in leaves for performance in fluctuating light conditions that are partially regulated by light-induced ion homeostatsis in the mesophyll cells. It is still elusive whether high light-induced leaf mesophyll ion fluxes affect leaf photosynthesis at different canopy levels in Solanum lycopersicum L. Leaf gas exchange and microelectrode ion flux (MIFE) measurements were employed to study the effects of prolonged light-induced canopy-level leaf physiological responses of tomato plants. High light resulted in a significant lowering in photosynthesis in the fully-exposed top canopy leaves of tomato, but not to mid- or low-canopy leaves. Leaf mesophyll K+ effluxes of all canopies were significantly decreased after three weeks of high light treatment. However, high light-induced leaf mesophyll Ca2+ effluxes were significantly enhanced only in the top and mid canopies. Moreover, we found that photosynthetic parameters were significantly correlated with leaf mesophyll ion fluxes. We thus propose that canopy-level significant Ca2+ efflux and K+ efflux of leaf mesophyll may serve as early indicators for light-induced regulation on photosynthesis. We conclude that light-induced differential photosynthetic performance and ion fluxes in leaves may implicate a requirement of more uniform light irradiance and spectra at different canopy levels of tall greenhouse tomato plants. This can be achieved through new innovative greenhouse lighting technologies and covering materials towards the enhancement of crop photosynthesis and yield.







Similar content being viewed by others
References
Adem GD, Chen G, Shabala L, Chen Z-H, Shabala S (2020) GORK channel: a master switch of plant metabolism? Trends Plant Sci 25:434–445
Aliniaeifard S, van Meeteren U (2013) Can prolonged exposure to low VPD disturb the ABA signalling in stomatal guard cells? J Exp Bot 64(12):3551–3566
Allorent G, Petroutsos D (2017) Photoreceptor-dependent regulation of photoprotection. Curr Opin Plant Biol 37:102–108
Armbruster U, Carrillo LR, Venema K, Pavlovic L, Schmidtmann E, Kornfeld A, Jahns P, Berry JA, Kramer DM, Jonikas MC (2014) Ion antiport accelerates photosynthetic acclimation in fluctuating light environments. Nat Commun 5:6439
Babla M, Cai S, Chen G, Tissue DT, Cazzonelli CI, Chen Z-H (2019) Molecular evolution and interaction of membrane transport and photoreception in plants. Front Genet 10:956
Babourina O, Newman I, Shabala S (2002) Blue light-induced kinetics of H+ and Ca2+ fluxes in etiolated wild-type and phototropin-mutant Arabidopsis seedlings. Proc Natl Acad Sci 99(4):2433–2438
Baum G, Long JC, Jenkins GI, Trewavas AJ (1999) Stimulation of the blue light phototropic receptor NPH1 causes a transient increase in cytosolic Ca2+. Proc Natl Acad Sci 96(23):13554–13559
Bellando M, Marre M, Sacco S, Talarico A, Venegoni A, Marre E (1995) Transmembrane potential-mediated coupling between H+ pump operation and K+ fluxes in Elodea densa leaves hyperpolarized by fusicoccin, light or acid load. Plant Cell Environ 18(9):963–976
Bielczynski LW, Łącki MK, Hoefnagels I, Gambin A, Croce R (2017) Leaf and plant age affects photosynthetic performance and photoprotective capacity. Plant Physiol 175(4):1634–1648
Blatt MR (2000) Cellular Signaling and Volume Control in Stomatal Movements in Plants. Ann Rev Cell Dev Biol 16(1):221–241
Boonman A, Anten NP, Dueck TA, Jordi WJ, van der Werf A, Voesenek LA, Pons TL (2006) Functional significance of shade-induced leaf senescence in dense canopies: an experimental test using transgenic tobacco. Am Nat 168(5):597–607
Bose J, Pottosin I, Shabala SSS, Palmgren MG, Shabala S (2011) Calcium efflux systems in stress signaling and adaptation in plants. Front Plant Sci 2:85
Brock TG, Cleland RE (1989) Role of acid efflux during growth promotion of primary leaves of Phaseolus vulgaris L. by hormones and light. Planta 177(4):476–482
Carmody M, Crisp PA, d’Alessandro S, Ganguly D, Gordon M, Havaux M, Albrecht-Borth V, Pogson BJ (2016) Uncoupling high light responses from singlet oxygen retrograde signaling and spatial-temporal systemic acquired acclimation. Plant Physiol 171(3):1734–1749
Chen Z, Newman I, Zhou M, Mendham N, Zhang G, Shabala S (2005) Screening plants for salt tolerance by measuring K+ flux: a case study for barley. Plant Cell Environ 28(10):1230–1246
Chen ZH, Hills A, Lim CK, Blatt MR (2010) Dynamic regulation of guard cell anion channels by cytosolic free Ca2+ concentration and protein phosphorylation. Plant J 61(5):816–825
Chen Z-H, Hills A, Bätz U, Amtmann A, Lew VL, Blatt MR (2012) Systems dynamic modeling of the stomatal guard cell predicts emergent behaviors in transport, signaling, and volume control. Plant Physiol 159(3):1235–1251
Davies JM, Poole RJ, Rea PA, Sanders D (1992) Potassium transport into plant vacuoles energized directly by a proton-pumping inorganic pyrophosphatase. Proc Natl Acad Sci 89(24):11701–11705
Demarsy E, Goldschmidt-Clermont M, Ulm R (2018) Coping with ‘dark sides of the sun’ through photoreceptor signaling. Trends Plant Sci 23(3):260–271
Demidchik V, Cuin TA, Svistunenko D, Smith SJ, Miller AJ, Shabala S, Sokolik A, Yurin V (2010) Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced cell death. J Cell Sci 123(9):1468–1479
Devireddy AR, Zandalinas SI, Gómez-Cadenas A, Blumwald E, Mittler R (2018) Coordinating the overall stomatal response of plants: rapid leaf-to-leaf communication during light stress. Sci Signal 11(518):eaam9514
Dietz K-J (2015) Efficient high light acclimation involves rapid processes at multiple mechanistic levels. J Exp Bot 66:2401–2414
Dodd AN, Kudla J, Sanders D (2010) The language of calcium signaling. Annu Rev Plant Biol 61:593–620
Dreyer I, Uozumi N (2011) Potassium channels in plant cells. FEBS J 278(22):4293–4303
Elzenga JTM, Van Volkenburgh E (1997) Characterization of a light-controlled anion channel in the plasma membrane of mesophyll cells of pea. Plant Physiol 113(4):1419–1426
Elzenga JTM, Prins HB, Van Volkenburgh E (1995) Light-induced membrane potential changes of epidermal and mesophyll cells in growing leaves of Pisum sativum. Planta 197(1):127–134
Evans JR (1989) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78(1):9–19
Fan X-X, Xu Z-G, Liu X-Y, Tang C-M, Wang L-W, Han X-l (2013) Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci Hort 153:50–55
Fankhauser C, Chory J (1997) Light control of plant development. Annu Rev Cell Dev Biol 13(1):203–229
Felle H (2001) pH: signal and messenger in plant cells. Plant Biol 3(06):577–591
Felle H, Bertl A (1986) Light-induced cytoplasmic pH changes and their interrelation to the activity of the electrogenic proton pump in Riccia fluitans. Biochim Biophys Acta Bioenerg 848(2):176–182
Feng X, Liu W, Qiu CW, Zeng F, Wang Y, Zhang G, Chen ZH, Wu F (2020) HvAKT2 and HvHAK1 confer drought tolerance in barley through enhanced leaf mesophyll H+ homeostasis. Plant Biotech J 18:1683–1696
Franco-Navarro JD, Rosales MA, Cubero-Font P, Calvo P, Álvarez R, Diaz-Espejo A, Colmenero-Flores JM (2019) Chloride as a macronutrient increases water-use efficiency by anatomically driven reduced stomatal conductance and increased mesophyll diffusion to CO2. Plant J 99(5):815–831
Galvão VC, Fankhauser C (2015) Sensing the light environment in plants: photoreceptors and early signaling steps. Curr Opin Neurobiol 34:46–53
Gobert A, Isayenkov S, Voelker C, Czempinski K, Maathuis FJ (2007) The two-pore channel TPK1 gene encodes the vacuolar K+ conductance and plays a role in K+ homeostasis. Proc Natl Acad Sci 104(25):10726–10731
Gordon MJ, Carmody ME, Albrecht V, Pogson B (2013) Systemic and local responses to repeated HL stress-induced retrograde signaling in Arabidopsis. Front Plant Sci 3:303
Gorecka M, Alvarez-Fernandez R, Slattery K, McAusland L, Davey PA, Karpinski S, Lawson T, Mullineaux PM (2014) Abscisic acid signalling determines susceptibility of bundle sheath cells to photoinhibition in high light-exposed Arabidopsis leaves. Philos Trans R Soc Lond B Biol Sci 369(1640):20130234
Grabov A, Blatt MR (1999) A steep dependence of inward-rectifying potassium channels on cytosolic free calcium concentration increase evoked by hyperpolarization in guard cells. Plant Physiol 119(1):277–288
Guo Z, Wang F, Xiang X, Ahammed GJ, Wang M, Onac E, Zhou J, Xia X, Shi K, Yin X (2016) Systemic induction of photosynthesis via illumination of the shoot apex is mediated sequentially by phytochrome B, auxin and hydrogen peroxide in tomato. Plant Physiol 172(2):1259–1272
Hansen U-P, Moldaenke C, Tabrizi H, Ramm D (1993) The effect of transthylakoid proton uptake on cytosolic pH and the imbalance of ATP and NAPDH/H+ production as measured by CO2-and light-induced depolarisation of the plasmalemma. Plant Cell Physiol 34(5):681–695
Harada A, Sakai T, Okada K (2003) Phot1 and phot2 mediate blue light-induced transient increases in cytosolic Ca2+ differently in Arabidopsis leaves. Proc Natl Acad Sci 100(14):8583–8588
Herdean A, Teardo E, Nilsson AK, Pfeil BE, Johansson ON, Ünnep R, Nagy G, Zsiros O, Dana S, Solymosi K (2016) A voltage-dependent chloride channel fine-tunes photosynthesis in plants. Nat Commun 7:11654
Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux P (1999) Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 284(5414):654–657
Kinoshita T, Nishimura M, Shimazaki K (1995) Cytosolic concentration of Ca2+ regulates the plasma membrane H+-ATPase in guard cells of fava bean. Plant Cell 7(8):1333–1342
Kirst H, Gabilly ST, Niyogi KK, Lemaux PG, Melis A (2017) Photosynthetic antenna engineering to improve crop yields. Planta 245(5):1009–1020
Kirst H, Shen Y, Vamvaka E, Betterle N, Xu D, Warek U, Strickland JA, Melis A (2018) Downregulation of the CpSRP43 gene expression confers a truncated light-harvesting antenna (TLA) and enhances biomass and leaf-to-stem ratio in Nicotiana tabacum canopies. Planta 248(1):139–154
Kromdijk J, Głowacka K, Leonelli L, Gabilly ST, Iwai M, Niyogi KK, Long SP (2016) Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354(6314):857–861
Lawson T, Vialet-Chabrand S (2019) Speedy stomata, photosynthesis and plant water use efficiency. New Phytol 221(1):93–98
Li Q, Kubota C (2009) Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ Exp Bot 67(1):59–64
Li Z, Wakao S, Fischer BB, Niyogi KK (2009) Sensing and responding to excess light. Annu Rev Plant Biol 60:239–260
Long S, Humphries S, Falkowski PG (1994) Photoinhibition of photosynthesis in nature. Annu Rev Plant Biol 45(1):633–662
López-Juez E, Bowyer JR, Sakai T (2007) Distinct leaf developmental and gene expression responses to light quantity depend on blue-photoreceptor or plastid-derived signals, and can occur in the absence of phototropins. Planta 227(1):113–123
Mak M, Babla M, Xu S-C, O’Carrigan A, Liu X-H, Gong Y-M, Holford P, Chen Z-H (2014) Leaf mesophyll K+, H+ and Ca2+ fluxes are involved in drought-induced decrease in photosynthesis and stomatal closure in soybean. Environ Exp Bot 98:1–12
Mak M, Zhang M, Randall D, Holford P, Milham P, Wu F, Zhang G, Chen Z-H (2019) Chloride transport at plant-soil Interface modulates barley cd tolerance. Plant Soil 441(1–2):409–421
Marre M, Albergoni F, Moroni A, Marre E (1989) Light-induced activation of electrogenic H+ extrusion and K+ uptake in Elodea densa depends on photosynthesis and is mediated by the plasma membrane H+ ATPase. J Exp Bot 40(3):343–352
McAinsh MR, Pittman JK (2009) Shaping the calcium signature. New Phytol 181(2):275–294
McAvoy RJ, Janes HW (1988) Alternative production strategies for greenhouse tomatoes using supplemental lighting. Sci Hortic 35(3–4):161–166
Melis A (2009) Solar energy conversion efficiencies in photosynthesis: minimizing the chlorophyll antennae to maximize efficiency. Plant Sci 177(4):272–280
Merilo E, Jalakas P, Kollist H, Brosché M (2015) The role of ABA recycling and transporter proteins in rapid stomatal responses to reduced air humidity, elevated CO2, and exogenous ABA. Mol Plant 8(4):657–659
Mühling KH, Läuchli A (2000) Light-induced pH and K+ changes in the apoplast of intact leaves. Planta 212(1):9–15
Newman I (2001) Ion transport in roots: measurement of fluxes using ion-selective microelectrodes to characterize transporter function. Plant Cell Environ 24(1):1–14
Niinemets U (2007) Photosynthesis and resource distribution through plant canopies. Plant Cell Environ 30(9):1052–1071
O’Carrigan A, Hinde E, Lu N, Xu X-Q, Duan H, Huang G, Mak M, Bellotti B, Chen Z-H (2013) Effects of light irradiance on stomatal regulation and growth of tomato. Environ Exp Bot 98:65–73
O’Carrigan A, Hinde E, Lu N, Xu X-Q, Duan H, Huang G, Mak M, Bellotti B, Chen Z-H (2014) Effects of light irradiance on stomatal regulation and growth of tomato. Environ Exp Bot 98:65–73
Peiter E, Maathuis FJM, Mills LN, Knight H, Pelloux J, Hetherington AM, Sanders D (2005) The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature 434(7031):404–408
Prins H, Snel J, Zanstra P, Helder R (1982) The mechanism of bicarbonate assimilation by the polar leaves of Potamogeton and Elodea. CO2 concentrations at the leaf surface. Plant Cell Environ 5(3):207–214
Rajapakse NC, Pollock RK, McMahon MJ, Kelly JW, Young RE (1992) Interpretation of light quality measurements and plant response in spectral filter research. HortSci 27(11):1208–1211
Raven JA (2014) Speedy small stomata? J Exp Bot 65(6):1415–1424
Rossel JB, Wilson PB, Hussain D, Woo NS, Gordon MJ, Mewett OP, Howell KA, Whelan J, Kazan K, Pogson BJ (2007) Systemic and intracellular responses to photooxidative stress in Arabidopsis. Plant Cell 19(12):4091–4110
Serrano R (1988) Structure and function of proton translocating ATPase in plasma membranes of plants and fungi. Biochim Biophys Acta Biomembr 947(1):1–28
Shabala S, Cuin TA (2008) Potassium transport and plant salt tolerance. Physiol Plant 133(4):651–669
Shabala S, Newman I (1999) Light-induced changes in hydrogen, calcium, potassium, and chloride ion fluxes and concentrations from the mesophyll and epidermal tissues of bean leaves. Understanding the ionic basis of light-induced bioelectrogenesis. Plant Physiol 119(3):1115–1124
Shabala S, Wilson S (2001) Fluctuations in light intensity modulate ion fluxes from grape berry mesocarp: direct evidence from microelectrode ion flux estimations. Aust J Grape Wine Res 7:137–143
Shabala SN, Newman IA, Morris J (1997) Oscillations in H+ and Ca2+ ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiol 113(1):111–118
Shabala S, Cuin TA, Prismall L, Nemchinov LG (2007) Expression of animal CED-9 anti-apoptotic gene in tobacco modifies plasma membrane ion fluxes in response to salinity and oxidative stress. Planta 227(1):189–197
Staal M, Elzenga JTM, van Elk AG, Prins HB, Van Volkenburgh E (1994) Red and blue light-stimulated proton efflux by epidermal leaf cells of the Argenteum mutant of Pisum sativum. J Exp Bot 45(9):1213–1218
Stahlberg R, Van Volkenburgh E (1999) The effect of light on membrane potential, apoplastic pH and cell expansion in leaves of Pisum sativum L. var. Argenteum. Planta 208(2):188–195
Steinger T, Roy B, Stanton M (2003) Evolution in stressful environments II: adaptive value and costs of plasticity in response to low light in Sinapis arvensis. J Evol Biol 16(2):313–323
Stewart D, Costa C, Dwyer L, Smith D, Hamilton R, Ma B (2003) Canopy structure, light interception, and photosynthesis in maize. Agron J 95(6):1465–1474
Stiles KA, McClintick A, Van Volkenburgh E (2003) A developmental gradient in the mechanism of K+ uptake during light-stimulated leaf growth in Nicotiana tabacum L. Planta 217(4):587–596
Stoelzle S, Kagawa T, Wada M, Hedrich R, Dietrich P (2003) Blue light activates calcium-permeable channels in Arabidopsis mesophyll cells via the phototropin signaling pathway. Proc Natl Acad Sci USA 100(3):1456–1461
Szabò I, Spetea C (2017) Impact of the ion transportome of chloroplasts on the optimization of photosynthesis. J Exp Bot 68(12):3115–3128
Taiz L, Zeiger E (2002) Photosynthesis: physiological and ecological considerations. Plant physiology, 3rd edn. Sinauer Associates, Sunderland, pp 171–192
Teakle NL, Tyerman SD (2010) Mechanisms of Cl- transport contributing to salt tolerance. Plant Cell Environ 33(4):566–589
Trouwborst G, Oosterkamp J, Hogewoning SW, Harbinson J, Van Ieperen W (2010) The responses of light interception, photosynthesis and fruit yield of cucumber to LED-lighting within the canopy. Physiol Plant 138(3):289–300
Urrestarazu M, Nájera C, del Mar GM (2016) Effect of the spectral quality and intensity of light-emitting diodes on several horticultural crops. HortSci 51(3):268–271
Volkenburgh EV (1999) Leaf expansion–an integrating plant behaviour. Plant Cell Environ 22(12):1463–1473
Wang F, Chen Z-H, Liu X, Colmer TD, Shabala L, Salih A, Zhou M, Shabala S (2016) Revealing the roles of GORK channels and NADPH oxidase in acclimation to hypoxia in Arabidopsis. J Exp Bot 68(12):3191–3204
Wang F, Chen Z-H, Shabala S (2017) Hypoxia sensing in plants: on a quest for ion channels as putative oxygen sensors. Plant Cell Physiol 58(7):1126–1142
Wilmer C, Fricker M (1996) Stomata. Chapmann & Hall, London
Xu H-L, Gauthier L, Desjardins Y, Gosselin A (1997) Photosynthesis in leaves, fruits, stem and petioles of greenhouse-grown tomato plants. Photosynthetica 33(1):113–123
Yong MT, Solis CA, Rabbi B, Huda S, Liu R, Zhou M, Shabala L, Venkataraman G, Shabala S, Chen ZH (2020) Leaf mesophyll K+ and Cl− fluxes and reactive oxygen species production predict rice salt tolerance at reproductive stage in greenhouse and field conditions. Plant Growth Regul 92:53–64
Zhang S, Ma K, Chen L (2003) Response of photosynthetic plasticity of Paeonia suffruticosa to changed light environments. Environ Exp Bot 49(2):121–133
Zhao C, Wang Y, Chan KX, Marchant DB, Franks PJ, Randall D, Tee EE, Chen G, Ramesh S, Phua SY (2019) Evolution of chloroplast retrograde signaling facilitates green plant adaptation to land. Proc Natl Acad Sci 116(11):5015–5020
Živanović B, Pang J, Shabala S (2005) Light-induced transient ion flux responses from maize leaves and their association with leaf growth and photosynthesis. Plant Cell Environ 28(3):340–352
Živanović BD, Cuin TA, Shabala S (2007) Spectral and dose dependence of light-induced ion flux responses from maize leaves and their involvement in leaf expansion growth. Plant Cell Physiol 48:598–605
Acknowledgements
We thank Linda Westmoreland, Sean Marney, Kim Nguyen, Renee Smith, and Rosemary Freeman for their technical assistance and maintenance of the glasshosue. ZHC is supported by Australian Research Council (DE1401011143) and Horticulture Innovation Australia (VG17003, LP18000).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Communicated by Anastasios Melis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Babla, M.H., Tissue, D.T., Cazzonelli, C.I. et al. Effect of high light on canopy-level photosynthesis and leaf mesophyll ion flux in tomato. Planta 252, 80 (2020). https://doi.org/10.1007/s00425-020-03493-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-020-03493-0