Abstract
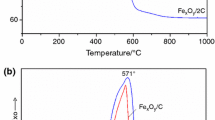
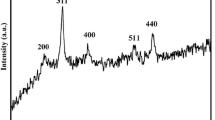
A green, non-polluting magnetic iron–carbon composite material FCH with humic acid as a raw material was synthesized by high-temperature oxidation–reduction method, and was first applied to the adsorption and removal of methylene blue in water. And it has lower synthesis cost. The morphology and microstructure of the samples were characterized by scanning electron microscopy, X-ray diffractometer, Thermal analysis, and Fourier transforms infrared spectroscopy was used to analyze the composition and structure of materials, and the magnetic strength of the samples was analyzed by using a vibrating sample magnetometer. The pseudo-first-order and pseudo-second-order kinetic models were used to describe the kinetic data and the Langmuir and Freundlich models were applied to describe the adsorption isotherms. The results showed that the equilibrium adsorption data were found to fit better to the Langmuir adsorption model and the kinetic process of adsorption could be described by the pseudo-first-order model. Compared with humic acid, iron–carbon composite materials can effectively improve the adsorption rate and adsorption capacity of methylene blue, and the adsorption capacity is as high as 34.45 mg/g within 30 min. Moreover, the material has super paramagnetic strength, and the composite material can be quickly separated from the aqueous solution in the presence of an external magnetic field. It finds that the material had good reusability through regeneration experiments.








Similar content being viewed by others
References
A. Sandoval, C. Hernández-Ventura, T.E. Klimova, Titanate nanotubes for removal of methylene blue dye by combined adsorption and photocatalysis. Fuel 198, 22–30 (2016)
L. Lefebvre, G. Agusti, A. Bouzeggane et al., Adsorption of dye with carbon media supported on polyurethane open cell foam. Catal. Today 301, 98–103 (2017)
F. Wang, L. Zhang, Y. Wang et al., Fe3O4@SiO2@CS-TETA functionalized graphene oxide for the adsorption of methylene blue (MB) and Cu (II). Appl. Surf. Sci. 420, 970–981 (2017)
B. Wang, B. Gao, A.R. Zimmerman et al., Impregnation of multiwall carbon nanotubes in alginate beads dramatically enhances their adsorptive ability to aqueous methylene blue. Chem. Eng. Res. Des. 133, 235–242 (2018)
L. Ai, L. Li, Efficient removal of organic dyes from aqueous solution with ecofriendly biomass-derived carbon@montmorillonite nanocomposites by one-step hydrothermal process. Chem. Eng. J. 223(3), 688–695 (2013)
Y. Cao, Z. Pan, Q. Shi et al., Modification of chitin with high adsorption capacity for methylene blue removal. Int. J. Biol. Macromol. 114, 392–399 (2018)
A.A. Narvekar, J.B. Fernandes, S.G. Tilve, Adsorption behavior of methylene blue on glycerol-based carbon materials. J. Environ. Chem. Eng. 6(2), 1714–1725 (2018)
O.S. Bello, I.A. Adeogun, J.C. Ajaelu et al., Adsorption of methylene blue onto activated carbon derived from periwinkle shells: kinetics and equilibrium studies. Chem. Ecol. 24(4), 285–295 (2008)
P. Chen, Z.F. Cao, X. Wen et al., In situ nano-silicate functionalized graphene oxide composites to improve MB removal. J. Taiwan Inst. Chem. Eng. 81, 87–94 (2017)
V. Katheresan, J. Kansedo, S.Y. Lau, Efficiency of various recent wastewater dye removal methods: a review. J. Environ. Chem. Eng. 6(4), 4676–4697 (2018)
S. Zereshki, P. Daraei, A. Shokri, Application of edible paraffin oil for cationic dye removal from water using emulsion liquid membrane. J. Hazard. Mater. 365, 1–8 (2018)
P. Chen, Z.F. Cao, S. Wang et al., In situ nano-silicate functionalized magnetic composites by (poly)dopamine to improve MB removal. Colloids Surf. A 552, 89–97 (2018)
X. Wang, C. Jiang, B. Hou et al., Carbon composite lignin-based adsorbents for the adsorption of dyes. Chemosphere 206, 587–596 (2018)
S.M. Stagnaro, C. Volzone, L. Huck, Nanoclay as adsorbent: evaluation for removing dyes used in the textile industry. Procedia Mater. Science 8, 586–591 (2015)
O. León, A. Muñoz-Bonilla, D. Soto et al., Removal of anionic and cationic dyes with bioadsorbent oxidized chitosans. Carbohydr. Polym. 194, 375–383 (2018)
L. Dai, F. Tan, H. Li et al., Calcium-rich biochar from the pyrolysis of crab shell for phosphorus removal. J. Environ. Manag. 198, 70–74 (2017)
S.F. Azha, L. Sellaoui, M.S. Shamsudin et al., Synthesis and characterization of a novel amphoteric adsorbent coating for anionic and cationic dyes adsorption: experimental investigation and statistical physics modelling. Chem. Eng. J. 351, 221–229 (2018)
K. He, G. Chen, G. Zeng et al., Enhanced removal performance for methylene blue by kaolin with graphene oxide modification. J. Taiwan Inst. Chem. Eng. 89, 77–85 (2018)
I. Hussain, Y. Li, J. Qi et al., Nitrogen-enriched carbon sheet for Methyl blue dye adsorption. J. Environ. Manag. 215, 123–131 (2018)
K. Omri, I. Najeh, L. El Mir, Influence of annealing temperature on the microstructure and dielectric properties of ZnO nanoparticles. Ceram. Int. 42(7), 8940–8948 (2016)
K. Omri, A. Bettaibi, K. Khirouni et al., The optoelectronic properties and role of Cu concentration on the structural and electrical properties of Cu doped ZnO nanoparticles. Phys. B 537, 167–175 (2018)
K. Omri, A. Alyamani, L. El Mir, Surface morphology, microstructure and electrical properties of Ca-doped ZnO thin films. J. Mater. Sci. 30, 16606–16612 (2019)
E. Sabah, S. Ouki, Sepiolite and sepiolite-bound humic acid interactions in alkaline media and the mechanism of the formation of sepiolite-humic acid complexes. Int. J. Miner. Process. 162, 69–80 (2017)
P. Jin, J. Song, L. Yang et al., Selective binding behavior of humic acid removal by aluminum coagulation. Environ. Pollut. 233(5), 290–298 (2017)
A. Manzak, C. Kurşun, Y. Yıldız, Characterization of humic acid extracted from aqueous solutions with polymer inclusion membranes. J. Taiwan Inst. Chem. Eng. 81, 14–20 (2017)
F. Labbé, E. Disa, Y. Ahmad et al., Tin dioxide coated carbon materials as an alternative catalyst support for PEMFCs: impacts of the intrinsic carbon properties and the synthesis parameters on the coating characteristics. Microporous Mesoporous Mater. 271, 1–15 (2018)
Z. Zhang, D. Niu, Y. Li et al., Magnetic, core-shell structured and surface molecularly imprinted polymers for the rapid and selective recognition of salicylic acid from aqueous solutions. Appl. Surf. Sci. 435, 178–186 (2018)
M. Anand, S.A. Farooqui, J. Singh et al., Mechanistic in-operando FT-IR studies for hydroprocessing of triglycerides. Catal. Today 309, 11–17 (2017)
Y.T. Ng, W. Kong, I. Kong, Magnetite-functionalized graphene nanohybrids: preparation and characterization of electrical and magnetic property. Mater. Today Proc. 5(1), 3202–3210 (2018)
D. Tong, C.W. Wu, M.O. Adebajo et al., Adsorption of methylene blue from aqueous solution onto porous cellulose-derived carbon/montmorillonite nanocomposites. Appl. Clay Sci. 61, 256–264 (2018)
Y. Wei, B. Han, X. Hu et al., Synthesis of Fe3O4 nanoparticles and their magnetic properties. Procedia Eng. 27, 632–637 (2012)
X. Liu, Y. Ma, Q. Zhang et al., Facile synthesis of Fe3O4/C composites for broadband microwave absorption properties. Appl. Surf. Sci. 445, 82–88 (2018)
S.Y. Nguang, R.W. Suzanna, S.L. Jia et al., Enhancing adsorption property of Engelhard Titanosilicate-10 through incorporation of graphene oxide. Microporous Mesoporous Mater. 252, 125–139 (2017)
M. Cheng, G. Zeng, D. Huang et al., High adsorption of methylene blue by salicylic acid-methanol modified steel converter slag and evaluation of its mechanism. J. Colloid Interface Sci. 515, 232–239 (2018)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, A., Sun, X., Li, B. et al. Preparation of Carbon–Iron Composites Materials and Studies of Its Adsorption Properties for the Methylene Blue. J Inorg Organomet Polym 31, 1293–1303 (2021). https://doi.org/10.1007/s10904-020-01754-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-020-01754-9