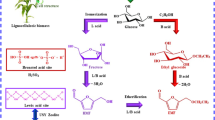
Abstract
Transforming biomass resources into high-quality liquid fuels is a very crucial, effective, and feasible approach in biorefinery processes. Among various biomass-derived liquid fuels, 2,5-bis(alkoxymethyl)furans (BAMFs), which can be produced from 2,5-bis(hydroxymethyl)furan (BHMF), 5-hydroxymethylfurfural (HMF), or fructose, are particularly attractive and widely considered to be a new type of biodiesel candidates or diesel additives due to their excellent physicochemical properties, such as high energy density, high cetane number, high boiling point, and strong stability, so they have received wide attention in recent years. At present, the relevant studies on the production of BAMFs are rapidly implementing, many progressive techniques are constantly developed and satisfactory results are increasingly obtained. However, up to now, a special, systematic and intensive review is still lacking in this research area. To better understand the current research situation, this review comprehensively summarizes and discusses different production methods and corresponding achievements of BAMFs, and emphatically analyzes the main functions and cooperative actions of active sites of catalysts in dehydration, reduction, and etherification reactions as well as the merits and demerits of exogenous and endogenous hydrogen systems. Moreover, this review also proposes several valuable and available ideas and viewpoints for the future studies of BAMFs. In a word, the main objective of this review is to draw more concerns about BAMFs and provide some theoretical references and technical supports for the high-efficiency, green, and economical production of BAMFs.

Graphical abstract










Similar content being viewed by others
References
Mika LT, Csefalvay E, Nemeth A (2018) Catalytic conversion of carbohydrates to initial platform chemicals: chemistry and sustainability. Chem Rev 118:505–613. https://doi.org/10.1021/acs.chemrev.7b00395
Chen S, Wojcieszak R, Dumeignil F, Marceau E, Royer S (2018) How catalysts and experimental conditions determine the selective hydroconversion of furfural and 5-hydroxymethylfurfural. Chem Rev 118:11023–11117. https://doi.org/10.1021/acs.chemrev.8b00134
Zhang ZR, Song JL, Han BX (2017) Catalytic transformation of lignocellulose into chemicals and fuel products in ionic liquids. Chem Rev 117:6834–6880. https://doi.org/10.1021/acs.chemrev.6b00457
Agarwal B, Kailasam K, Sangwan RS, Elumalai S (2018) Traversing the history of solid catalysts for heterogeneous synthesis of 5-hydroxymethylfurfural from carbohydrate sugars: a review. Renew Sust Energ Rev 82:2408–2425. https://doi.org/10.1016/j.rser.2017.08.088
Hu L, Lin L, Wu Z, Zhou SY, Liu SJ (2017) Recent advances in catalytic transformation of biomass-derived 5-hydroxymethylfurfural into the innovative fuels and chemicals. Renew Sust Energ Rev 74:230–257. https://doi.org/10.1016/j.rser.2017.02.042
Kong X, Zhu YF, Fang Z, Kozinski JA, Butler IS, Xu L, Song H, Wei XJ (2018) Catalytic conversion of 5-hydroxymethylfurfural to some value-added derivatives. Green Chem 20:3657–3682. https://doi.org/10.1039/c8gc00234g
Luo X, Li Y, Gupta NK, Sels B, Ralph J, Shuai L (2020) Protection strategies enable selective conversion of biomass. Angew Chem Int Ed 59:11704–11716. https://doi.org/10.1002/anie.201914703
Hu L, Xu JX, Zhou SY, He AY, Tang X, Lin L, Xu JM, Zhao YJ (2018) Catalytic advances in the production and application of biomass-derived 2,5-dihydroxymethylfuran. ACS Catal 8:2959–2980. https://doi.org/10.1021/acscatal.7b03530
Graham BJ, Raines RT (2018) Efficient metal-free conversion of glucose to 5-hydroxymethylfurfural using a boronic acid. Biomass Conv Bioref 9:471–477. https://doi.org/10.1007/s13399-018-0346-2
Xu Y, Liu G, Fu J, Kang S, Xiao Y, Yang P, Liao W (2019) Catalytic hydrolysis of cellulose to levulinic acid by partly replacing sulfuric acid with Nafion® NR50 catalyst. Biomass Conv Bioref 9:609–616. https://doi.org/10.1007/s13399-019-00373-w
Bungay HR (1982) Biomass refining. Science 218:643–646. https://doi.org/10.1126/science.218.4573.643
Pileidis FD, Titirici MM (2016) Levulinic acid biorefineries: new challenges for efficient utilization of biomass. ChemSusChem 9:562–582. https://doi.org/10.1002/cssc.201501405
Esposito D, Antonietti M (2015) Redefining biorefinery: the search for unconventional building blocks for materials. Chem Soc Rev 44:5821–5835. https://doi.org/10.1039/c4cs00368c
Liu DJ, Chen EYX (2014) Organocatalysis in biorefining for biomass conversion and upgrading. Green Chem 16:964–981. https://doi.org/10.1039/c3gc41934g
Karinen R, Vilonen K, Niemela M (2011) Biorefining: heterogeneously catalyzed reactions of carbohydrates for the production of furfural and hydroxymethylfurfural. ChemSusChem 4:1002–1016. https://doi.org/10.1002/cssc.201000375
Ma JP, Shi S, Jia X, Xia F, Ma H, Gao J, Xu J (2019) Advances in catalytic conversion of lignocellulose to chemicals and liquid fuels. J Energy Chem 36:74–86. https://doi.org/10.1016/j.jechem.2019.04.026
Li H, Riisager A, Saravanamurugan S, Pandey A, Sangwan RS, Yang S, Luque R (2018) Carbon-increasing catalytic strategies for upgrading biomass into enery-intensive fuels and chemicals. ACS Catal 8:148–187. https://doi.org/10.1021/acscatal.7b02577
Asomaning J, Haupt S, Chae M, Bressler DC (2018) Recent developments in microwave-assisted thermal conversion of biomass for fuels and chemicals. Renew Sust Energ Rev 92:642–657. https://doi.org/10.1016/j.rser.2018.04.084
Shylesh S, Gokhale AA, Ho CR, Bell AT (2017) Novel strategies for the production of fuels, lubricants, and chemicals from biomass. Acc Chem Res 50:2589–2597. https://doi.org/10.1021/acs.accounts.7b00354
Li H, Fang Z, Smith RL, Yang S (2016) Efficient valorization of biomass to biofuels with bifunctional solid catalytic materials. Prog Energy Combust Sci 55:98–194. https://doi.org/10.1016/j.pecs.2016.04.004
Natsir TA, Shimazu S (2020) Fuels and fuel additives from furfural derivatives via etherification and formation of methylfurans. Fuel Process Technol 200:106308. https://doi.org/10.1016/j.fuproc.2019.106308
Liu H, Tang X, Hao WW, Zeng XH, Sun Y, Lei TZ, Lin L (2018) One-pot tandem conversion of fructose into biofuel components with in-situ generated catalyst system. J Energy Chem 27:375–380. https://doi.org/10.1016/j.jechem.2018.01.002
Wei JN, Wang T, Liu H, Li MZ, Tang X, Sun Y, Zeng XH, Hu L, Lei TZ, Lin L (2019) Highly efficient reductive etherification of 5-hydroxymethylfurfural to 2,5-bis(alkoxymethyl)furans as biodiesel components over Zr-SBA catalyst. Energ Technol 7:1801071. https://doi.org/10.1002/ente.201801071
Wei JN, Wang T, Cao XJ, Liu H, Tang X, Sun Y, Zeng XH, Lei TZ, Liu SJ, Lin L (2019) A flexible Cu-based catalyst system for the transformation of fructose to furanyl ethers as potential bio-fuels. Appl Catal B Environ 258:117793. https://doi.org/10.1016/j.apcatb.2019.117793
Nguyen H, Xiao N, Daniels S, Marcella N, Timoshenko J, Frenkel A, Vlachos DG (2017) Role of Lewis and Brønsted acidity in metal chloride catalysis in organic media: reductive etherification of furanics. ACS Catal 7:7363–7370. https://doi.org/10.1021/acscatal.7b02348
Wei JN, Cao XJ, Wang T, Liu H, Tang X, Zeng XH, Sun Y, Lei TZ, Liu SJ, Lin L (2018) Catalytic transfer hydrogenation of biomass-derived 5-hydroxymethylfurfural into 2,5-bis(hydroxymethyl)furan over tunable Zr-based bimetallic catalyst. Catal Sci Technol 8:4474–4484. https://doi.org/10.1039/C8CY00500A
De Jong E, Vijlbrief T, Hijkoop R, Gruter GJM, Van Der Waal JC (2012) Promising results with YXY diesel components in an ESC test cycle using a PACCAR diesel engine. Biomass Bioenergy 36:151–159. https://doi.org/10.1016/j.biombioe.2011.10.034
Cao Q, Liang WY, Guan J, Wang L, Qu Q, Zhang XZ, Wang XC, Mu XD (2014) Catalytic synthesis of 2,5-bis-methoxymethylfuran: a promising cetane number improver for diesel. Appl Catal A Gen 481:49–53. https://doi.org/10.1016/j.apcata.2014.05.003
Fang WT, Hu HL, Dong P, Ma ZS, He YL, Wang L, Zhang YJ (2018) Improvement of furanic diether selectivity by adjusting Brønsted and Lewis acidity. Appl Catal A Gen 565:146–151. https://doi.org/10.1016/j.apcata.2018.07.013
Balakrishnan M, Sacia ER, Bell AT (2012) Etherification and reductive etherification of 5-(hydroxymethyl)furfural: 5-(Alkoxymethyl)furfurals and 2,5-bis(alkoxymethyl)furans as potential bio-diesel candidates. Green Chem 14:1626–1634. https://doi.org/10.1039/c2gc35102a
Han J, Kim J, Jung BY, Hwang S, Jegal J, Kim YH, Lee YS (2017) Highly selective catalytic hydrogenation and etherification of 5-hydroxymethyl-2-furaldehyde to 2,5-bis(alkoxymethyl)furans for potential biodiesel production. Synlett 28:2299–2302. https://doi.org/10.1055/s-0036-1589076
Musolino M, Ginés-Molina MJ, Moreno-Tost R, Aricò F (2019) Purolite-catalyzed etherification of 2,5-bis(hydroxymethyl)furan: a systematic study. ACS Sustain Chem Eng 7:10221–10226. https://doi.org/10.1021/acssuschemeng.9b01413
Guo W, Heeres HJ, Yue J (2020) Continuous synthesis of 5-hydroxymethylfurfural from glucose using a combination of AlCl3 and HCl as catalyst in a biphasic slug flow capillary microreactor. Chem Eng J 381:122754. https://doi.org/10.1016/j.cej.2019.122754
Qiu G, Huang C, Sun X, Chen B (2019) Highly active niobium-loaded montmorillonite catalysts for the production of 5-hydroxymethylfurfural from glucose. Green Chem 21:3930–3939. https://doi.org/10.1039/c9gc01225g
Huang FM, Su YW, Long ZY, Chen GJ, Yao Y (2018) Enhanced formation of 5-hydroxymethylfurfural from glucose using a silica-supported phosphate and iron phosphate heterogeneous catalyst. Ind Eng Chem Res 57:10198–10205. https://doi.org/10.1021/acs.iecr.8b01531
Chen DW, Liang FB, Feng DX, Xian M, Zhang HB, Liu HZ, Du FL (2016) An efficient route from reproducible glucose to 5-hydroxymethylfurfural catalyzed by porous coordination polymer heterogeneous catalysts. Chem Eng J 300:177–184. https://doi.org/10.1016/j.cej.2016.04.039
Huang B, Cheng Y, Ma J, Chen Z, Yu K, Sun Y (2019) The titanium-aluminum binary oxide immobilized over long-axis SBA-15 as efficient and benign catalyst for conversion of sucrose into 5-hydroxymethylfurfural. Catal Surv Jpn 23:181–198. https://doi.org/10.1007/s10563-019-09267-3
Qiu G, Wang XC, Huang CP, Zhang P, Li YX, Chen BH (2018) Synthesis of 5-hydroxymethylfurfural from sucrose catalyzed by phosphotungstate. Chin J Org Chem 38:940–948. https://doi.org/10.6023/cjoc201709007
Yu SB, Zang HJ, Yang XL, Zhang MC, Xie RR, Yu PF (2017) Highly efficient preparation of 5-hydroxymethylfurfural from sucrose using ionic liquids and heteropolyacid catalysts in dimethyl sulfoxide-water mixed solvent. Chin Chem Lett 28:1479–1484. https://doi.org/10.1016/j.cclet.2017.02.016
Kreissl HT, Nakagawa K, Peng YK, Koito Y, Zheng JL, Tsang SCE (2016) Niobium oxides: correlation of acidity with structure and catalytic performance in sucrose conversion to 5-hydroxymethylfurfural. J Catal 338:329–339. https://doi.org/10.1016/j.jcat.2016.03.007
Li H, Yang S (2014) Catalytic transformation of fructose and sucrose to HMF with proline-derived ionic liquids under mild conditions. Int J Chem Eng 2014:1–7. https://doi.org/10.1155/2014/978708
Kimura H, Yoshida K, Uosaki Y, Nakahara M (2013) Effect of water content on conversion of cellobiose into 5-hydroxymethyl-2-furaldehyde in a dimethyl sulfoxide-water mixture. J Phys Chem A 117:10987–10996. https://doi.org/10.1021/jp407801u
Beckerle K, Okuda J (2012) Conversion of glucose and cellobiose into 5-hydroxymethylfurfural (HMF) by rare earth metal salts in N,N′-dimethylacetamide (DMA). J Mol Catal A Chem 356:158–164. https://doi.org/10.1016/j.molcata.2012.01.008
Hu L, Zhao G, Tang X, Wu Z, Xu JX, Lin L, Liu SJ (2013) Catalytic conversion of carbohydrates into 5-hydroxymethylfurfural over cellulose-derived carbonaceous catalyst in ionic liquid. Bioresour Technol 148:501–507. https://doi.org/10.1016/j.biortech.2013.09.016
Zhang L, Shah A, Michel FC (2019) Synthesis of 5-hydroxymethylfurfural from fructose and inulin catalyzed by magnetically-recoverable Fe3O4@SiO2@TiO2-HPW nanoparticles. J Chem Technol Biotechnol 94:3393–3402. https://doi.org/10.1002/jctb.6153
Xie HB, Zhao ZB, Wang Q (2012) Catalytic conversion of inulin and fructose into 5-hydroxymethylfurfural by lignosulfonic acid in ionic liquids. ChemSusChem 5:901–905. https://doi.org/10.1002/cssc.201100588
Shen X, Wang YX, Hu CW, Qian K, Ji Z, Jin M (2012) One-pot conversion of inulin to furan derivatives catalyzed by sulfated TiO2/mordenite solid acid. ChemCatChem 4:2013–2019. https://doi.org/10.1002/cctc.201200190
Yi YB, Lee JW, Hong SS, Choi YH, Chung CH (2011) Acid-mediated production of hydroxymethylfurfural from raw plant biomass with high inulin in an ionic liquid. J Ind Eng Chem 17:6–9. https://doi.org/10.1016/j.jiec.2010.12.017
Wu S, Fan H, Xie Y, Cheng Y, Wang Q, Zhang Z, Han BX (2010) Effect of CO2 on conversion of inulin to 5-hydroxymethylfurfural and propylene oxide to 1,2-propanediol in water. Green Chem 12:1215–1219. https://doi.org/10.1039/c002553d
Matharu AS, Ahmed S, Almonthery B, Macquarrie DJ, Lee YS, Kim Y (2018) Starbon/high-amylose corn starch-supported N-heterocyclic carbene-iron(III) catalyst for conversion of fructose into 5-hydroxymethylfurfural. ChemSusChem 11:716–725. https://doi.org/10.1002/cssc.201702207
Cao L, Yu IKM, Tsang DCW, Zhang S, Ok YS, Kwon EE, Song H, Poon CS (2018) Phosphoric acid-activated wood biochar for catalytic conversion of starch-rich food waste into glucose and 5-hydroxymethylfurfural. Bioresour Technol 267:242–248. https://doi.org/10.1016/j.biortech.2018.07.048
Yu IK, Tsang DC, Yip AC, Chen SS, Wang L, Ok YS, Poon CS (2017) Catalytic valorization of starch-rich food waste into hydroxymethylfurfural (HMF): controlling relative kinetics for high productivity. Bioresour Technol 237:222–230. https://doi.org/10.1016/j.biortech.2017.01.017
Goswami SR, Dumont MJ, Raghavan V (2016) Microwave assisted synthesis of 5-hydroxymethylfurfural from starch in AlCl3·6H2O/DMSO/[BMIM]Cl system. Ind Eng Chem Res 55:4473–4481. https://doi.org/10.1021/acs.iecr.6b00201
Yepez A, Garcia A, Climent MS, Romero Reyes AA, Luque R (2014) Catalytic conversion of starch into valuable furan derivatives using supported metal nanoparticles on mesoporous aluminosilicate materials. Catal Sci Technol 4:428–434. https://doi.org/10.1039/c3cy00762f
Fang J, Zheng W, Liu K, Li H, Li C (2020) Molecular design and experimental study on the synergistic catalysis of cellulose into 5-hydroxymethylfurfural with Brønsted-Lewis acidic ionic liquids. Chem Eng J 385:123796. https://doi.org/10.1016/j.cej.2019.123796
Cao Z, Fan ZX, Chen Y, Li M, Shen T, Zhu CJ, Ying HJ (2019) Efficient preparation of 5-hydroxymethylfurfural from cellulose in a biphasic system over hafnyl phosphates. Appl Catal B Environ 244:170–177. https://doi.org/10.1016/j.apcatb.2018.11.019
Li XC, Peng KH, Xia QN, Liu XH, Wang YQ (2018) Efficient conversion of cellulose into 5-hydroxymethylfurfural over niobia/carbon composites. Chem Eng J 332:528–536. https://doi.org/10.1016/j.cej.2017.06.105
Zhang C, Cheng ZT, Fu ZH, Liu YC, Yi XF, Zheng AM, Kirk SR, Yin DL (2016) Effective transformation of cellulose to 5-hydroxymethylfurfural catalyzed by fluorine anion-containing ionic liquid modified biochar sulfonic acids in water. Cellulose 24:95–106. https://doi.org/10.1007/s10570-016-1118-4
Atanda L, Konarova M, Ma Q, Mukundan S, Shrotri A, Beltramini J (2016) High yield conversion of cellulosic biomass into 5-hydroxymethylfurfural and a study of the reaction kinetics of cellulose to HMF conversion in a biphasic system. Catal Sci Technol 6:6257–6266. https://doi.org/10.1039/c6cy00820h
Yu X, Chu Y, Zhang L, Shi H, Xie M, Peng L, Guo X, Li W, Xue N, Ding W (2020) Adjacent acid sites cooperatively catalyze fructose to 5-hydroxymethylfurfural in a new, facile pathway. J Energy Chem 47:112–117. https://doi.org/10.1016/j.jechem.2019.11.020
Johnson RL, Perras FA, Hanrahan MP, Mellmer M, Garrison TF, Kobayashi T, Dumesic JA, Pruski M, Rossini AJ, Shanks BH (2019) Condensed phase deactivation of solid Brønsted acids in the dehydration of fructose to hydroxymethylfurfural. ACS Catal 9:11568–11578. https://doi.org/10.1021/acscatal.9b03455
He YX, Itta AK, Alwakwak A-a, Huang M, Rezaei F, Rownaghi AA (2018) Aminosilane-grafted SiO2-ZrO2 polymer hollow fibers as bifunctional microfluidic reactor for tandem reaction of glucose and fructose to 5-hydroxymethylfurfural. ACS Sustain Chem Eng 6:17211–17219. https://doi.org/10.1021/acssuschemeng.8b04555
Yang ZZ, Qi W, Huang RL, Fang J, Su RX, He ZM (2016) Functionalized silica nanoparticles for conversion of fructose to 5-hydroxymethylfurfural. Chem Eng J 296:209–216. https://doi.org/10.1016/j.cej.2016.03.084
Li XL, Zhang K, Chen SY, Li C, Li F, Xu HJ, Fu Y (2018) A cobalt catalyst for reductive etherification of 5-hydroxymethylfurfural to 2,5-bis(methoxymethyl)furan under mild conditions. Green Chem 20:1095–1105. https://doi.org/10.1039/c7gc03072j
Sacia ER, Balakrishnan M, Bell AT (2014) Biomass conversion to diesel via the etherification of furanyl alcohols catalyzed by Amberlyst-15. J Catal 313:70–79. https://doi.org/10.1016/j.jcat.2014.02.012
Fang W, Hu H, Ma Z, Wang L, Zhang Y (2018) Two possible side reaction pathways during furanic etherification. Catalysts 8:383–392. https://doi.org/10.3390/catal8090383
Hu H, Hu D, Jin H, Zhang P, Li G, Zhou H, Yang Y, Chen C, Zhang J, Wang L (2019) Efficient production of furanic diether in a continuous fixed bed reactor. ChemCatChem 11:2179–2186. https://doi.org/10.1002/cctc.201900054
Gupta D, Saha B (2018) Dual acidic titania carbocatalyst for cascade reaction of sugar to etherified fuel additives. Catal Commun 110:46–50. https://doi.org/10.1016/j.catcom.2018.02.026
Elsayed I, Jackson MA, Hassan EB (2020) Hydrogen-free catalytic reduction of biomass-derived 5-hydroxymethylfurfural into 2,5-bis(hydroxymethyl)furan using copper-iron oxides bimetallic catalyst. ACS Sustain Chem Eng 8:1774–1785. https://doi.org/10.1021/acssuschemeng.9b05575
Li H, Yang TT, Fang Z (2018) Biomass-derived mesoporous Hf-containing hybrid for efficient Meerwein-Ponndorf-Verley reduction at low temperatures. Appl Catal B Environ 227:79–89. https://doi.org/10.1016/j.apcatb.2018.01.017
Pasini T, Lolli A, Albonetti S, Cavani F, Mella M (2014) Methanol as a clean and efficient H-transfer reactant for carbonyl reduction: scope, limitations, and reaction mechanism. J Catal 317:206–219. https://doi.org/10.1016/j.jcat.2014.06.023
Op De Beeck B, Dusselier M, Geboers J, Holsbeek J, Morré E, Oswald S, Giebeler L, Sels BF (2015) Direct catalytic conversion of cellulose to liquid straight-chain alkanes. Energy Environ Sci 8:230–240. https://doi.org/10.1039/c4ee01523a
Gao Z, Fan GL, Yang L, Li F (2017) Double-active sites cooperatively catalyzed transfer hydrogenation of ethyl levulinate over a ruthenium-based catalyst. Mol Catal 442:181–190. https://doi.org/10.1016/j.mcat.2017.09.026
Li AY, Segalla A, Li CJ, Moores A (2017) Mechanochemical metal-free transfer hydrogenation of carbonyls using polymethylhydrosiloxane as the hydrogen source. ACS Sustain Chem Eng 5:11752–11760. https://doi.org/10.1021/acssuschemeng.7b03298
Zhou S, Dai F, Chen Y, Dang C, Zhang C, Liu D, Qi H (2019) Sustainable hydrothermal self-assembly of hafnium-lignosulfonate nanohybrids for highly efficient reductive upgrading of 5-hydroxymethylfurfural. Green Chem 21:1421–1431. https://doi.org/10.1039/c8gc03710h
Xue ZM, Jiang JY, Li GF, Zhao WC, Wang JF, Mu TC (2016) Zirconium-cyanuric acid coordination polymer: highly efficient catalyst for conversion of levulinic acid to γ-valerolactone. Catal Sci Technol 6:5374–5379. https://doi.org/10.1039/C5CY02215K
Xiang XM, Cui JL, Ding GQ, Zheng HY, Zhu YL, Li YW (2016) One-step continuous conversion of fructose to 2,5-dihydroxymethylfuran and 2,5-dimethylfuran. ACS Sustain Chem Eng 4:4506–4510. https://doi.org/10.1021/acssuschemeng.6b01411
Upare PP, Hwang YK, Hwang DW (2018) An integrated process for the production of 2,5-dihydroxymethylfuran and its polymer from fructose. Green Chem 20:879–885. https://doi.org/10.1039/c7gc03597g
Thananatthanachon T, Rauchfuss TB (2010) Efficient route to hydroxymethylfurans from sugars via transfer hydrogenation. ChemSusChem 3:1139–1141. https://doi.org/10.1002/cssc.201000209
Wu D, Hernández WY, Zhang S, Vovk EI, Zhou X, Yang Y, Khodakov AY, Ordomsky VV (2019) In situ generation of Brønsted acidity in the Pd-I bifunctional catalysts for selective reductive etherification of carbonyl compounds under mild conditions. ACS Catal 9:2940–2948. https://doi.org/10.1021/acscatal.8b04925
Leng Y, Shi L, Du S, Jiang J, Jiang P (2020) A tannin-derived zirconium-containing porous hybrid for efficient Meerwein-Ponndorf-Verley reduction under mild conditions. Green Chem 22:180–186. https://doi.org/10.1039/c9gc03393a
Zhou S, Dai F, Xiang Z, Song T, Liu D, Lu F, Qi H (2019) Zirconium-lignosulfonate polyphenolic polymer for highly efficient hydrogen transfer of biomass-derived oxygenates under mild conditions. Appl Catal B Environ 248:31–43. https://doi.org/10.1016/j.apcatb.2019.02.011
Rojas-Buzo S, Garcia-Garcia P, Corma A (2018) Catalytic transfer hydrogenation of biomass-derived carbonyls over hafnium-based metal-organic frameworks. ChemSusChem 11:432–438. https://doi.org/10.1002/cssc.201701708
Li H, He J, Riisager A, Saravanamurugan S, Song B, Yang S (2016) Acid-base bifunctional zirconium N-alkyltriphosphate nanohybrid for hydrogen transfer of biomass-derived carboxides. ACS Catal 6:7722–7727. https://doi.org/10.1021/acscatal.6b02431
Hu L, Li N, Dai XL, Guo YQ, Jiang YT, He AY, Xu JX (2019) Highly efficient production of 2,5-dihydroxymethylfuran from biomass-derived 5-hydroxymethylfurfural over an amorphous and mesoporous zirconium phosphonate catalyst. J Energy Chem 37:82–92. https://doi.org/10.1016/j.jechem.2018.12.001
Hu L, Dai XL, Li N, Tang X, Jiang YT (2019) Highly selective hydrogenation of biomass-derived 5-hydroxymethylfurfural into 2,5-bis(hydroxymethyl)furan over an acid–base bifunctional hafnium-based coordination polymer catalyst. Sustainable Energy Fuels 3:1033–1041. https://doi.org/10.1039/c8se00545a
Hu L, Li T, Xu JX, He AY, Tang X, Chu XZ, Xu JM (2018) Catalytic transfer hydrogenation of biomass-derived 5-hydroxymethylfurfural into 2,5-dihydroxymethylfuran over magnetic zirconium-based coordination polymer. Chem Eng J 352:110–119. https://doi.org/10.1016/j.cej.2018.07.007
Hu D, Hu H, Jin H, Zhang P, Hu Y, Ying S, Li X, Yang Y, Zhang J, Wang L (2020) Building hierarchical zeolite structure by post-synthesis treatment to promote the conversion of furanic molecules into biofuels. Appl Catal A Gen 590:117338. https://doi.org/10.1016/j.apcata.2019.117338
Jae J, Mahmoud E, Lobo RF, Vlachos DG (2014) Cascade of liquid-phase catalytic transfer hydrogenation and etherification of 5-hydroxymethylfurfural to potential biodiesel components over Lewis acid zeolites. ChemCatChem 6:508–513. https://doi.org/10.1002/cctc.201300978
Luo J, Yu JY, Gorte RJ, Mahmoud E, Vlachos DG, Smith MA (2014) The effect of oxide acidity on HMF etherification. Catal Sci Technol 4:3074–3081. https://doi.org/10.1039/c4cy00563e
Lewis JD, Van de Vyver S, Crisci AJ, Gunther WR, Michaelis VK, Griffin RG, Roman-Leshkov Y (2014) A continuous flow strategy for the coupled transfer hydrogenation and etherification of 5-(hydroxymethyl)furfural using Lewis acid zeolites. ChemSusChem 7:2255–2265. https://doi.org/10.1002/cssc.201402100
Shinde S, Rode CV (2017) Cascade reductive-etherification of bio-derived aldehydes over Zr-based catalysts. ChemSusChem 10:4090–4101. https://doi.org/10.1002/cssc.201701275
Huang RL, Qi W, Su RX, He ZM (2010) Integrating enzymatic and acid catalysis to convert glucose into 5-hydroxymethylfurfural. Chem Commun 46:1115–1117. https://doi.org/10.1039/b921306f
Qi XH, Watanabe M, Aida TM, Smith RL (2010) Fast transformation of glucose and di-/polysaccharides into 5-hydroxymethylfurfural by microwave heating in an ionic liquid/catalyst system. ChemSusChem 3:1071–1077. https://doi.org/10.1002/cssc.201000124
Hu L, Sun Y, Lin L (2012) Efficient conversion of glucose into 5-hydroxymethylfurfural by chromium(III) chloride in inexpensive ionic liquid. Ind Eng Chem Res 51:1099–1104. https://doi.org/10.1021/ie202174f
Liu B, Zhang ZH, Zhao ZB (2013) Microwave-assisted catalytic conversion of cellulose into 5-hydroxymethylfurfural in ionic liquids. Chem Eng J 215-216:517–521. https://doi.org/10.1016/j.cej.2012.11.019
Galkin KI, Ananikov VP (2019) When will 5-hydroxymethylfurfural, the “sleeping giant” of sustainable chemistry, awaken? ChemSusChem 12:2976–2982. https://doi.org/10.1002/cssc.201900592
Zhang Y, Li B, Wei Y, Yan C, Meng M, Yan Y (2019) Direct synthesis of metal-organic frameworks catalysts with tunable acid-base strength for glucose dehydration to 5-hydroxymethylfurfural. J Taiwan Inst Chem Eng 96:93–103. https://doi.org/10.1016/j.jtice.2018.12.020
Wang HL, Kong QQ, Wang YX, Deng TS, Chen CM, Hou XL, Zhu YL (2014) Graphene oxide catalyzed dehydration of fructose into 5-hydroxymethylfurfural with isopropanol as cosolvent. ChemCatChem 6:728–732. https://doi.org/10.1002/cctc.201301067
Hu L, Tang X, Wu Z, Lin L, Xu JX, Xu N, Dai BL (2015) Magnetic lignin-derived carbonaceous catalyst for the dehydration of fructose into 5-hydroxymethylfurfural in dimethylsulfoxide. Chem Eng J 263:299–308. https://doi.org/10.1016/j.cej.2014.11.044
Sun Q, Tang Y, Aguila B, Wang S, Xiao FS, Thallapally PK, Alenizi AM, Nafady A, Ma S (2019) Reaction environment modification in covalent organic frameworks for catalytic performance enhancement. Angew Chem Int Ed 131:8762–8767. https://doi.org/10.1002/anie.201900029
Marullo S, Rizzo C, D’Anna F (2019) Activity of a heterogeneous catalyst in deep eutectic solvents: the case of carbohydrate conversion into 5-hydroxymethylfurfural. ACS Sustain Chem Eng 7:13359–13368. https://doi.org/10.1021/acssuschemeng.9b02605
Wei J, Wang T, Liu H, Liu Y, Tang X, Sun Y, Zeng X, Lei T, Liu S, Lin L (2020) Assembly of Zr-based coordination polymer over USY zeolite as a highly efficient and robust acid catalyst for one-pot transformation of fructose into 2,5-bis(isopropoxymethyl)furan. J Catal 389:87–98. https://doi.org/10.1016/j.jcat.2020.05.020
Funding
This work was sponsored by the Natural Science Foundation of Jiangsu Province (BK20190105 and BK20191056), the National Natural Science Foundation of China (22078123 and 21908075), the Industry-Academia Cooperation Project of Jiangsu Province (BY2019150), the Natural Science Foundation of the Higher Education Institutions of Jiangsu Province (19KJA150010 and 19KJB22006), the Natural Science Foundation of Huaian City (HAB202057), the Qinglan Project of Jiangsu Province, the Youth Talent Promotion Project of Jiangsu Association of Science and Technology, and the Open Project of Jiangsu Key Laboratory for Biomass-based Energy and Enzyme Technology (BEETKC1908).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, L., Jiang, Y., Wang, X. et al. Recent advances and mechanistic insights on the production of biomass-derived 2,5-bis(alkoxymethyl)furans. Biomass Conv. Bioref. 13, 1343–1358 (2023). https://doi.org/10.1007/s13399-020-01062-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01062-9