Abstract
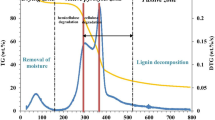
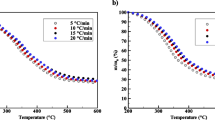
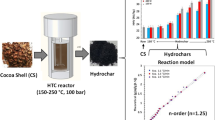
This study presents the first attempt to focus on the cocoa shell pyrolysis in terms of kinetic triplet, thermodynamic parameters, and evolved volatile analysis using the TGA-FTIR technique. For reliable interpretation of the multistep pyrolysis of cocoa shell, the multiple kinetic triplets were adequately estimated by a combined kinetic procedure using five independent parallel reactions with the Vyazovkin isoconversional method, the compensation effect, and the master plot method. According to the results, the multiple kinetic triplets were able to describe the pyrolysis behavior of the cocoa shell with an accuracy of R2 > 0.9446 and the pyrolysis mechanisms exhibited different reaction models (n-order and contracting cylinder). Evolved volatile analysis suggested the presence of high-energy compounds (aromatic) and useful chemicals (aldehyde, ketone, esters, ether, and alcohols). The pre-exponential factors for the five pseudo-components of cocoa shell pyrolysis ranged from 2.56 × 1011 to 8.66 × 1016 min−1 (derived from the compensation effect method), while the values of Ea ranged from 99 to 271 kJ mol−1. From a comparative analysis, it was found that the results from the compensation effect method ensured the overall kinetic expression to be consistent with the experimental cocoa shell pyrolysis behavior. In contrast, the overall kinetic expression using pre-exponential factors derived from Kissinger’s method failed to match the experimental curves. The pyrolytic conversion of cocoa shell into bioenergy appeared as potentially viable (Ea – ΔH ≤ 5.5 kJ mol−1). The promising findings on the cocoa shell pyrolysis can expand the use of this residue in bioenergy applications, consisting of a great attempt toward its valorization.

Graphical abstract






Similar content being viewed by others
References
FAOSTAT (2020) Cocoa Beans (Crops Production). Food and agriculture organization of the United Nations (Statistics Division). Available: <http://www.fao.org/faostat/en/#data>. (Accessed 10 February 2020)
Vásquez ZS, de Carvalho Neto DP, Pereira GVM, Vandenberghe LPS, de Oliveira PZ, Tiburcio PB, Rogez HLG, Góes Neto A, Soccol CR (2019) Biotechnological approaches for cocoa waste management: a review. Waste Manag 90:72–83. https://doi.org/10.1016/j.wasman.2019.04.030
Alves JLF, Da Silva JCG, da Silva Filho VF et al (2019) Determination of the bioenergy potential of Brazilian pine-fruit shell via pyrolysis kinetics, thermodynamic study, and evolved gas analysis. Bioenergy Res 12:168–183. https://doi.org/10.1007/s12155-019-9964-1
Chen J, Wang Y, Lang X, Ren X', Fan S (2017) Evaluation of agricultural residues pyrolysis under non-isothermal conditions: thermal behaviors, kinetics, and thermodynamics. Bioresour Technol 241:340–348. https://doi.org/10.1016/j.biortech.2017.05.036
Fernandez A, Ortiz LR, Asensio D, Rodriguez R, Mazza G (2020) Kinetic analysis and thermodynamics properties of air/steam gasification of agricultural waste. J Environ Chem Eng 8:103829. https://doi.org/10.1016/j.jece.2020.103829
Alves JLF, Da Silva JCG, Di Domenico M et al (2020) Exploring Açaí seed (Euterpe oleracea) pyrolysis using multi-component kinetics and thermodynamics assessment towards its bioenergy potential. BioEnergy Res. https://doi.org/10.1007/s12155-020-10175-y
Vyazovkin S, Wight CA (1999) Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim Acta 340–341:53–68. https://doi.org/10.1016/S0040-6031(99)00253-1
da Silva JCG, de Albuquerque JG, Galdino WV d A et al (2020) Single-step and multi-step thermokinetic study – deconvolution method as a simple pathway for describe properly the biomass pyrolysis for energy conversion. Energy Convers Manag 209:112653. https://doi.org/10.1016/j.enconman.2020.112653
ASTM (2018) E698-18: Kinetic parameters for thermally unstable materials using differential scanning calorimetry and the Flynn/Wall/Ozawa method. In: Annual Book of ASTM Standards. ASTM International, West Conshohocken, pp 1–9. https://doi.org/10.1520/E0698-18
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N (2011) ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta 520:1–19. https://doi.org/10.1016/j.tca.2011.03.034
da Silva JCG, Alves JLF, Galdino WV d A et al (2018) Pyrolysis kinetic evaluation by single-step for waste wood from reforestation. Waste Manag 72:265–273. https://doi.org/10.1016/j.wasman.2017.11.034
Papadikis K, Gu S, Bridgwater AV, Gerhauser H (2009) Application of CFD to model fast pyrolysis of biomass. Fuel Process Technol 90:504–512. https://doi.org/10.1016/j.fuproc.2009.01.010
Ranganathan P, Gu S (2015) Computational fluid dynamics modelling of biomass fast pyrolysis in fluidised bed reactors, focusing different kinetic schemes. Bioresour Technol 213:333–341. https://doi.org/10.1016/j.biortech.2016.02.042
Boateng AA, Mtui PL (2012) CFD modeling of space-time evolution of fast pyrolysis products in a bench-scale fluidized-bed reactor. Appl Therm Eng 33–34:190–198. https://doi.org/10.1016/j.applthermaleng.2011.09.034
Mumbach GD, Alves JLF, Da Silva JCG et al (2019) Thermal investigation of plastic solid waste pyrolysis via the deconvolution technique using the asymmetric double sigmoidal function: determination of the kinetic triplet, thermodynamic parameters, thermal lifetime and pyrolytic oil composition for clean. Energy Convers Manag 200:112031. https://doi.org/10.1016/j.enconman.2019.112031
Alves JLF, Da Silva JCG, da Silva Filho VF et al (2019) Bioenergy potential of red macroalgae Gelidium floridanum by pyrolysis: evaluation of kinetic triplet and thermodynamics parameters. Bioresour Technol 291:121892. https://doi.org/10.1016/j.biortech.2019.121892
Lopez-Velazquez MA, Santes V, Balmaseda J, Torres-Garcia E (2013) Pyrolysis of orange waste: a thermo-kinetic study. J Anal Appl Pyrolysis 99:170–177. https://doi.org/10.1016/j.jaap.2012.09.016
Janković B, Manić N, Dodevski V, Popović J, Rusmirović JD, Tošić M (2019) Characterization analysis of Poplar fluff pyrolysis products. Multi-component kinetic study. Fuel 238:111–128. https://doi.org/10.1016/j.fuel.2018.10.064
Janković B, Manić N, Stojiljković D, Jovanović V (2018) TSA-MS characterization and kinetic study of the pyrolysis process of various types of biomass based on the Gaussian multi-peak fitting and peak-to-peak approaches. Fuel 234:447–463. https://doi.org/10.1016/j.fuel.2018.07.051
Wang X, Hu M, Hu W, Chen Z, Liu S, Hu Z, Xiao B (2016) Thermogravimetric kinetic study of agricultural residue biomass pyrolysis based on combined kinetics. Bioresour Technol 219:510–520. https://doi.org/10.1016/j.biortech.2016.07.136
Van de Velden M, Baeyens J, Brems A et al (2010) Fundamentals, kinetics and endothermicity of the biomass pyrolysis reaction. Renew Energy 35:232–242. https://doi.org/10.1016/j.renene.2009.04.019
ASTM (2014) E1131-08: Standard test method for compositional analysis by thermogravimetry. In: Annual Book of ASTM Standards. ASTM International, West Conshohocken, pp 1–6. https://doi.org/10.1520/E1131-08R14
ASTM (2008) D5373-08: Standard test methods for instrumental determination of carbon, hydrogen, and nitrogen in laboratory samples of coal. In: Annual Book of ASTM Standards. ASTM International, West Conshohocken, pp 1–9. https://doi.org/10.1520/D5373-08
ASTM (2017) D4239-17: Standard test method for sulfur in the analysis sample of coal and coke using high-temperature tube furnace combustion. In: Annual Book of ASTM Standards. West Conshohocken, pp 1–7. https://doi.org/10.1520/D4239-17
Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74:3583–3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2
Alves JLF, da Silva JCG, Mumbach GD, Domenico MD, de Sena RF, Machado RAF, Marangoni C (2020) Demonstrating the suitability of tamarind residues to bioenergy exploitation via combustion through physicochemical properties, performance indexes, and emission characteristics. BioEnergy Res. https://doi.org/10.1007/s12155-020-10158-z
ASTM (2013) D5865-13: Standard test method for gross calorific value of coal and coke. In: Annual Book of ASTM Standards. ASTM International, West Conshohocken, pp 1–19. https://doi.org/10.1520/D5865-13
ASTM (2019) E873-82: Standard test method for bulk density of densified particulate biomass fuels. In: Annual Book of ASTM Standards. ASTM International, West Conshohocken, pp 1–2. https://doi.org/10.1520/E0873-82R19
Kim YS, Kim YS, Kim SH (2010) Investigation of thermodynamic parameters in the thermal decomposition of plastic waste-waste lube oil compounds. Environ Sci Technol 44:5313–5317. https://doi.org/10.1021/es101163e
Frau C, Ferrara F, Orsini A, Pettinau A (2015) Characterization of several kinds of coal and biomass for pyrolysis and gasification. Fuel 152:138–145. https://doi.org/10.1016/j.fuel.2014.09.054
García R, Pizarro C, Lavín AG, Bueno JL (2012) Characterization of Spanish biomass wastes for energy use. Bioresour Technol 103:249–258. https://doi.org/10.1016/j.biortech.2011.10.004
Kalkreuth W, Holz M, Kern M, Machado G, Mexias A, Silva MB, Willett J, Finkelman R, Burger H (2006) Petrology and chemistry of Permian coals from the Paraná Basin: 1. Santa Terezinha, Leão-Butiá and Candiota Coalfields, Rio Grande do Sul, Brazil. Int J Coal Geol 68:79–116. https://doi.org/10.1016/j.coal.2005.10.006
Mythili R, Venkatachalam P, Subramanian P, Uma D (2013) Characterization of bioresidues for biooil production through pyrolysis. Bioresour Technol 138:71–78. https://doi.org/10.1016/j.biortech.2013.03.161
Titiloye JO, Abu Bakar MS, Odetoye TE (2013) Thermochemical characterisation of agricultural wastes from West Africa. Ind Crops Prod 47:199–203. https://doi.org/10.1016/j.indcrop.2013.03.011
Merdun H, Sezgin İV (2018) Products distribution of catalytic co-pyrolysis of greenhouse vegetable wastes and coal. Energy 162:953–963. https://doi.org/10.1016/j.energy.2018.08.004
Ceylan S, Topçu Y (2014) Pyrolysis kinetics of hazelnut husk using thermogravimetric analysis. Bioresour Technol 156:182–188. https://doi.org/10.1016/j.biortech.2014.01.040
Biagini E, Fantei A, Tognotti L (2008) Effect of the heating rate on the devolatilization of biomass residues. Thermochim Acta 472:55–63. https://doi.org/10.1016/j.tca.2008.03.015
Yuan T, Tahmasebi A, Yu J (2015) Comparative study on pyrolysis of lignocellulosic and algal biomass using a thermogravimetric and a fixed-bed reactor. Bioresour Technol 175:333–341. https://doi.org/10.1016/j.biortech.2014.10.108
Chen Z, Hu M, Zhu X, Guo D, Liu S, Hu Z, Xiao B, Wang J, Laghari M (2015) Characteristics and kinetic study on pyrolysis of five lignocellulosic biomass via thermogravimetric analysis. Bioresour Technol 192:441–450. https://doi.org/10.1016/j.biortech.2015.05.062
Várhegyi G (2007) Aims and methods in non-isothermal reaction kinetics. J Anal Appl Pyrolysis 79:278–288. https://doi.org/10.1016/j.jaap.2007.01.007
Xu Y, Chen B (2013) Investigation of thermodynamic parameters in the pyrolysis conversion of biomass and manure to biochars using thermogravimetric analysis. Bioresour Technol 146:485–493. https://doi.org/10.1016/j.biortech.2013.07.086
Kaur R, Gera P, Jha MK, Bhaskar T (2018) Pyrolysis kinetics and thermodynamic parameters of castor (Ricinus communis) residue using thermogravimetric analysis. Bioresour Technol 250:422–428. https://doi.org/10.1016/j.biortech.2017.11.077
Yang X, Zhao Y, Li R, Wu Y, Yang M (2018) A modified kinetic analysis method of cellulose pyrolysis based on TG–FTIR technique. Thermochim Acta 665:20–27. https://doi.org/10.1016/j.tca.2018.05.008
Yeo JY, Chin BLF, Tan JK, Loh YS (2017) Comparative studies on the pyrolysis of cellulose, hemicellulose, and lignin based on combined kinetics. J Energy Inst. 92:27–37. https://doi.org/10.1016/j.joei.2017.12.003
Chen C, Miao W, Zhou C, Wu H (2017) Thermogravimetric pyrolysis kinetics of bamboo waste via Asymmetric Double Sigmoidal (Asym2sig) function deconvolution. Bioresour Technol 225:48–57. https://doi.org/10.1016/j.biortech.2016.11.013
Pinzi S, Buratti C, Bartocci P, Marseglia G, Fantozzi F, Barbanera M (2020) A simplified method for kinetic modeling of coffee silver skin pyrolysis by coupling pseudo-components peaks deconvolution analysis and model free- isoconversional methods. Fuel 278:118260. https://doi.org/10.1016/j.fuel.2020.118260
Sharma P, Pandey OP, Diwan PK (2019) Non-isothermal kinetics of pseudo-components of waste biomass. Fuel 253:1149–1161. https://doi.org/10.1016/j.fuel.2019.05.093
Müsellim E, Tahir MH, Ahmad MS, Ceylan S (2018) Thermokinetic and TG/DSC-FTIR study of pea waste biomass pyrolysis. Appl Therm Eng 137:54–61. https://doi.org/10.1016/j.applthermaleng.2018.03.050
Ahmad MS, Mehmood MA, Taqvi STH, Elkamel A, Liu CG, Xu J, Rahimuddin SA, Gull M (2017) Pyrolysis, kinetics analysis, thermodynamics parameters and reaction mechanism of Typha latifolia to evaluate its bioenergy potential. Bioresour Technol 245:491–501. https://doi.org/10.1016/j.biortech.2017.08.162
Alhumade H, da Silva JCG, Ahmad MS, Çakman G, Yıldız A, Ceylan S, Elkamel A (2019) Investigation of pyrolysis kinetics and thermal behavior of Invasive Reed Canary (Phalaris arundinacea)for bioenergy potential. J Anal Appl Pyrolysis 140:385–392. https://doi.org/10.1016/j.jaap.2019.04.018
Konwar K, Nath HP, Bhuyan N, Saikia BK, Borah RC, Kalita AC, Saikia N (2019) Effect of biomass addition on the devolatilization kinetics, mechanisms and thermodynamics of a northeast Indian low rank sub-bituminous coal. Fuel 256:115926. https://doi.org/10.1016/j.fuel.2019.115926
Ming X, Xu F, Jiang Y, Zong P, Wang B, Li J, Qiao Y, Tian Y (2020) Thermal degradation of food waste by TG-FTIR and Py-GC/MS: pyrolysis behaviors, products, kinetic and thermodynamic analysis. J Clean Prod 244:118713. https://doi.org/10.1016/j.jclepro.2019.118713
Vyazovkin S, Burnham AK, Favergeon L, Koga N, Moukhina E, Pérez-Maqueda LA, Sbirrazzuoli N (2020) ICTAC Kinetics Committee recommendations for analysis of multi-step kinetics. Thermochim Acta 689:178597. https://doi.org/10.1016/j.tca.2020.178597
Wang S, Dai G, Yang H, Luo Z (2017) Lignocellulosic biomass pyrolysis mechanism: a state-of-the-art review. Prog Energy Combust Sci 62:33–86. https://doi.org/10.1016/j.pecs.2017.05.004
Gao N, Li A, Quan C, du L, Duan Y (2013) TG-FTIR and Py-GC/MS analysis on pyrolysis and combustion of pine sawdust. J Anal Appl Pyrolysis 100:26–32. https://doi.org/10.1016/j.jaap.2012.11.009
Fournel S, Marcos B, Godbout S, Heitz M (2015) Predicting gaseous emissions from small-scale combustion of agricultural biomass fuels. Bioresour Technol 179:165–172. https://doi.org/10.1016/j.biortech.2014.11.100
Tahir MH, Çakman G, Goldfarb JL, Topcu Y, Naqvi SR, Ceylan S (2019) Demonstrating the suitability of canola residue biomass to biofuel conversion via pyrolysis through reaction kinetics, thermodynamics and evolved gas analyses. Bioresour Technol 279:67–73. https://doi.org/10.1016/j.biortech.2019.01.106
Daugaard DE, Brown RC (2003) Enthalpy for pyrolysis for several types of biomass. Energy and Fuels 17:934–939. https://doi.org/10.1021/ef020260x
Funding
The authors acknowledge the financial support from the Brazilian Council for Scientific and Technological Development (CNPq/Brazil Process 423869/2016-7) and Brazilian Coordination for the Improvement of Higher Education Personnel (CAPES/Brazil Finance Code 001). This research was developed in LCP/UFSC, LCA/UFPB, and LabMaq/UFPB facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 72.3 kb)
Rights and permissions
About this article
Cite this article
Mumbach, G.D., Alves, J.L.F., da Silva, J.C.G. et al. Pyrolysis of cocoa shell and its bioenergy potential: evaluating the kinetic triplet, thermodynamic parameters, and evolved gas analysis using TGA-FTIR. Biomass Conv. Bioref. 12, 723–739 (2022). https://doi.org/10.1007/s13399-020-01058-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01058-5