Use of Small Cetaceans as Bait in Small-Scale Fisheries in Peru
- 1Centre for Ecology and Conservation, University of Exeter, Penryn, United Kingdom
- 2ProDelphinus, Lima, Peru
- 3Facultad de Biología Marina, Universidad Científica del Sur, Lima, Peru
The use of small cetaceans as bait is a practice that has been reported worldwide, affecting the conservation status of vulnerable species. In Peru specifically, it has been documented since at least the late 1990s. Here we document the various contemporary uses of small cetaceans, including targeted capture for subsequent use as fishing bait. We designed a survey addressing fishery characteristics, bycatch and the use as bait of small cetaceans, and the history of these practice. We surveyed 147 fishers based in the four Peruvian ports of Paita, Salaverry, Pucusana, and Ilo and held in-depth interviews with 12 fishers from Salaverry and Pucusana. Results from our surveys show that the majority of fishers have had small cetacean bycatch while fishing and that bycaught individuals in gillnets are commonly found dead (Salaverry: 100% of fishers, Pucusana: 58%) whereas in longlines small cetaceans are found alive (Paita: 74%, Ilo: 53%). We found that the use of dolphins as bait is still common in both gillnet and longline shark fisheries along the coast of Peru and that it is more frequent in northern ports. Gillnet fishers reported using one to four dolphins as bait per trip (10–15 sets) from bycatch events and discarding the rest if they have excessive bycatch, while longline fishers reported using 10–20 dolphins per fishing trip from either direct take by harpooning or the exchange of carcasses from gillnet vessels. Bycatch and use as bait mainly affects four species, the dusky, bottlenose and common dolphins and the Burmeister’s porpoise. We identified three drivers of the use of dolphins as bait: effectiveness, availability and cost. These factors will have to be addressed in parallel if this practice is to be reduced. We recommend combining legislative and community-led strategies to reduce bait use and thus further the conservation of small cetacean populations in the southeastern Pacific Ocean.
Introduction
For decades, the primary focus of research assessing interactions of small cetaceans (i.e., dolphins and porpoises) with fisheries has been on bycatch (Read et al., 2006; Read, 2008; Davidson et al., 2012; Reeves et al., 2013). However, the use of small cetaceans as bait is also a product of fisheries interactions (Mangel et al., 2010; Rosa et al., 2012; Mintzer et al., 2018). This use, which is illegal in most places (Ross, 2006; Mangel et al., 2010; Barbosa-Filho et al., 2016) is a widespread activity, particularly in developing countries, where socioeconomic factors motivate fishers to seek a bait that is effective, fresh and inexpensive or free (Cosentino and Fisher, 2016; Mintzer et al., 2018). This practice has been reported in Latin America for marine and also for freshwater cetacean species such as the Amazon river dolphin (Inia geoffrensis) and the tucuxi (Sotalia fluviatilis), for the piracatinga (Calophysus macropterus) fishery in Brazil, Colombia and Peru (Mintzer et al., 2013; Brum et al., 2015; Campbell et al., 2020). For marine species, their use has mostly been reported associated with shark fisheries, specifically in Colombia, Guatemala, and Peru (Avila et al., 2008; Mangel et al., 2010; Quintana-Rizzo, 2014).
In Peru, fishery interactions with small cetaceans have been documented since at least the 1960s, in particular with small-scale fisheries (Clarke, 1962; Read et al., 1988; Van Waerebeek and Reyes, 1990). Monitoring of landings in ports and fish markets from 1990 to 1993 estimated that the small cetacean catch reached 15,000–20,000 individuals per year, mostly used as a food source (Van Waerebeek and Reyes, 1994). Concern over these high numbers of captures led to legislative changes in 1996 that prohibited the capture, use, consumption and trade of dolphins and porpoises (Law No. 26585, 9 April 1996). Despite this ban, the use of dolphins as bait in Peru was first reported for longline shark fisheries in 1994 (Van Waerebeek and Reyes, 1994). This practice later extended to gillnets and spread to ports along the north and central Peru coast (Van Waerebeek et al., 1999). Given the challenges in assessing an illegal activity that occurs at sea far from any kind of enforcement officers, Mangel et al. (2010) used onboard observers to monitor small cetacean interactions on small-scale net vessels and estimated that 27% of monitored fishing trips from the port of Salaverry used small cetaceans for bait that were obtained from either bycatch or harpooning. While this study provided important information on a previously unreported, illegal use of cetaceans for one port, the extent of this practice along the Peruvian coast remains unclear and generating this information is challenging due to the nature of the practice. Given the size of the small-scale fleet in Peru and the conservation status of certain small cetacean species, the magnitude and extent of this practice should be factored into population estimates and conservation measures.
The study of cetaceans typically requires long and costly periods of fieldwork (Poonian et al., 2008; Braulik et al., 2018). The use of surveys and interviews is an alternative method, which has the advantages of being relatively fast and less costly than traditional methods (Moore et al., 2010; Braulik et al., 2018). This method has been successful in generating information on dolphins and the effect of ecotourism (Walpole and Goodwin, 2010; Cegarra and Pacheco, 2017), on community perceptions toward dolphins and other species (Dowling, 1993; Dawson et al., 2004; Scott and Parsons, 2005) as well as in generating information on interactions with fisheries (Avila et al., 2008; Antunes Zappes et al., 2013; Quintana-Rizzo, 2014).
Using surveys and interviews with individual fishers, the aim of this research was to investigate the use as bait of dolphins and porpoises in the Peruvian small-scale longline and gillnet shark fisheries. We specifically aimed to (1) better understand the fisheries at locations where cetaceans are used as bait, (2) determine if there is still a targeted fishery for small cetaceans, and (3) generate an estimate of how many individual small cetaceans are used in this practice.
Materials and Methods
The study was implemented in four Peruvian ports: Paita (5°10′1.42″S, 81°3′19.8″W), Salaverry (8°13′27″S, 78°58′35″W), Pucusana (12°28′54″S, 76°47′51″W), and Ilo (17°38′55″S, 71°19′50″W) (Figure 1), from December 2016 to January 2017 and September to November 2018. These four ports were chosen given their past and possible current use of dolphins as bait, as well as having active elasmobranch fisheries using gillnets and longlines (Alfaro-Shigueto et al., 2010; Doherty et al., 2014; Gonzalez-Pestana et al., 2016).
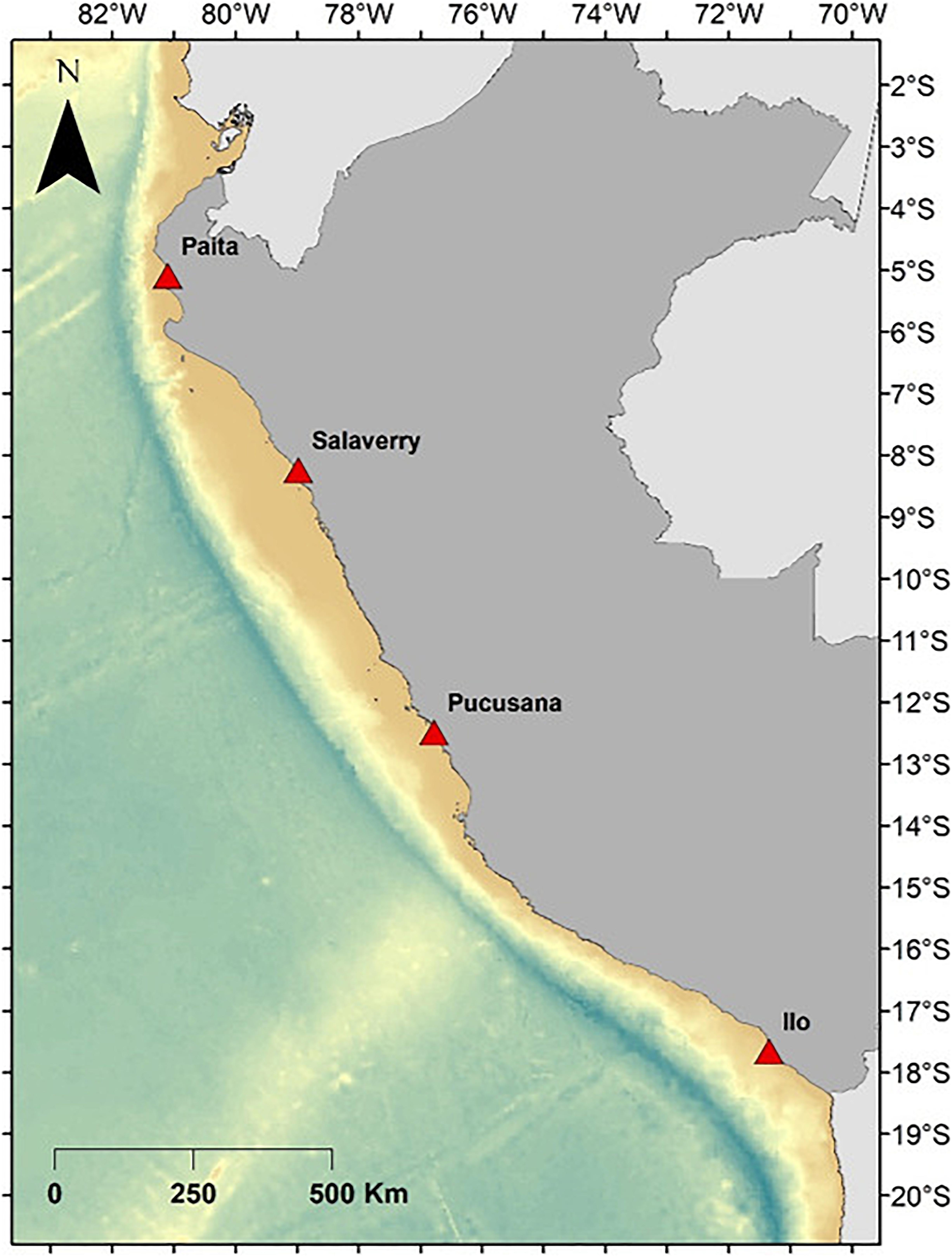
Figure 1. Peruvian coastline with the four ports in which surveys were conducted. Salaverry and Pucusana were revisited for in-depth interviews.
Surveys
Surveys were conducted with fishers who live and fish at the sampled sites. All participants were small-scale fishers that target elasmobranchs. Small-scale fishers are defined by the Peruvian government as using vessels with a maximum length of 15 m, a maximum storage capacity of 32.6 m3, and relying predominantly on manual labor (Ley General de Pesca, 2001). The sample size was calculated based on the number of vessels registered at each landing point provided by port-based personnel of marine government institutions to target a certain proportion of all fishers in a given port (Ministry of Production, Institute of Marine Investigation IMARPE). We surveyed fishers representative of a minimum of 20% of the fishing fleet per port. We interviewed by referral sampling method (Biernacki and Waldorf, 1981), one fisher per vessel to reduce possible duplication of information and interviewed only pelagic drifting gillnet and longline fishers. Research protocols were approved by the University of Exeter Ethics Committee (2016/1432).
The survey instrument (Supplementary Figure 1) included 36 questions on different themes including (1) characteristics of fishing activity and fishing effort, (2) bycatch and use of small cetaceans as bait, (3) and a historical section about the directed catch and use of small cetaceans. The questions were multiple choice when applicable and were conducted privately, anonymously and voluntarily. Each survey was initiated with a brief introduction about the study, the project objectives and the duration of the survey. If fishers consented to participate, we continued with the survey. Each survey took approximately 30 min to complete. In the four study areas, fishing is a job mostly taken by men. We did not identify women fishers in the sites sampled.
To analyze survey results, we used a generalized linear model (GLM) with a quasibinomial distribution, to account for overdispersion (Richards, 2008; R Core Team, 2017). For this model Y was the response variable extracted from the survey responses representing dolphin bait use (equivalent to 1) or no-use (equivalent to 0) for each case. The explanatory variables considered were port, fishing gear, number of years fishing, target catch, and bycatch (yes/no). We followed backward model selection, the best model was chosen by considering significant variables (p-value < 0.05) and comparing the QAIC values between models, where the smallest QAIC was considered the most appropriate (Burnham and Anderson, 2004). Once the best fitting model was chosen, we used the predict function, type “response” to estimate the probability of dolphin bait use.
In-Depth Individual Interviews
In Salaverry and Pucusana, we identified fishers that completed the surveys who also consented to participate in a more in-depth interview about the use of small cetaceans as bait. We identified these fishers by asking members of fisher associations and staff of marine government institutions who the most respected peer fishers or leaders of the fishing community were in each port. The interview did not have a structured list of questions, but rather a list of topics including: (1) the availability and illegal commerce of small cetaceans; (2) the characteristics of small cetacean carcass use (e.g., use in fishing gear, number of individuals needed for a fishing trip); and (3) the reasons why fishers use or might stop using small cetaceans as bait and possible alternatives to small cetacean bait. Written notes were taken during the individual interviews.
Results
Surveys
A total of 147 surveys from four fishing ports were completed (Paita: n = 41, Salaverry: n = 28, Pucusana: n = 24, Ilo: n = 54; Table 1).
Characteristics of Fishing Activity and Effort
Participating fishers were 22–78 years of age (average 43 years, SD = 10.8 years) and had an average of 25 years of fishing experience (SD = 12.0 years). In Paita and Ilo most participating fishermen used longlines (100 and 91%, respectively). In Salaverry, most participants used gillnets (84%). Pucusana had a mix of both fishing gears, with 48% of the fishers reporting using gillnets and 52% using longlines. Most fishermen operated year-round (SA: 82%, PU: 70%, ILO: 53%) with these gears, except in Paita where the longline fishery was seasonal (90%, December–March). Fishers reported that they conducted one (PA:85% SA:11% PU: 33% ILO: 19%) or two fishing trips per month (PA:8%, SA:71%, PU: 38%, ILO: 43%).
Bycatch and Use of Small Cetaceans as Bait
Most of the participating fishers confirmed seeing small cetaceans in their fishing zones (PA:100% SA: 96% PU: 96% ILO: 98%) and had a positive perception to seeing them during their fishing activities (PA:100% SA:79% PU: 75% ILO: 94%). The majority of fishers confirmed having had small cetacean bycatch while fishing (PA:50% SA:100% PU: 71% ILO: 56%) with an average of three animals reported bycaught per trip (Table 2).
Fishers from Salaverry and Pucusana described that bycaught dolphins and porpoises were usually found dead from drowning in the gear (SA: 100%, PU: 58%). In Paita and Ilo, where longlines were the most frequently used gear, respondents reported that dolphins are mostly found entangled alive (PA: 74%, ILO: 53%). Most fishers said that when small cetaceans are found alive, the animals are released (PA: 95%, SA: 82%, PU: 100%, ILO: 85%). More than half (PA: 38%, SA: 74%, PU: 68%, ILO: 56%) of the fishers in all ports reported that in cases where the dolphins were recovered dead, they were discarded at sea. Following “discard at sea,” the most frequent responses were the reported use of small cetaceans as bait (PA: 27%, SA: 17%, PU: 5%, ILO: 9%) and the use of dolphins for consumption (PA: 31%, SA: 6%, PU: 26%, ILO: 28%, Table 2).
We asked fishers if dolphins were used as bait in a second question that was open-ended. Fishers were asked to enumerate their favorite types of bait, in terms of effectiveness. Some fishers from the ports of Salaverry (33%, n = 9) and Pucusana (8%, n = 2) indicated that the bait that works best for shark fishing is dolphin meat. Other species mentioned as preferred bait were Pacific squid (Dosidicus gigas) (PA: 100%, SA: 17%, PU: 39%, ILO: 94%), mackerel (Scomber japonicus) (PA: 0%, SA: 17%, PU: 11%, ILO: 0%), and “tamborin” (Auxis spp.) (PA: 0%, SA: 8%, PU: 21%, ILO: 0%). Participating fishers sometimes declined to answer certain questions on the use of bait, however, some did respond about the preferred species of small cetaceans for use as bait (16% of total participants, n = 24). Half of the interviewed fishermen (50%) mentioned that they do not have a preference of small cetacean species to use as bait. From those fishermen who responded with a preference (n = 12), three species were noted as used for bait, the common dolphin (Delphinus sp., n = 5), dusky dolphin (Lagenorhynchus obscurus, n = 4), and bottlenose dolphin (Tursiops truncatus, n = 3). No fishers mentioned the use of or preference for Burmeister’s porpoises (Phocoena spinipinnis) as bait. We asked fishers how many individuals they use as bait per trip, and 14% of respondents (n = 20) provided a response. Of these, nine fishers responded that whatever species caught was used (n = 9), or 1 individual (n = 3), 2 individuals (n = 5). The remainder of the answers were from 3 to 5 individuals (n = 3).
Historical Use and Directed Catch of Dolphins
In the second portion of the survey, we asked fishers about the historical use of dolphins as bait. In Pucusana and Salaverry (SA: 44%, PU: 30%), respondents indicated that dolphins were hunted from fishing boats originating from their port in the past. When asked if this practice of direct catch of small cetaceans persisted, some fishers responded in the affirmative (Salaverry 25%, n = 7; Pucusana 8%, n = 2). In Paita and Ilo the majority said that this had not occurred in the past (PA: 78% n = 32, ILO: 74% n = 40). The majority of fishers responded that directed catches (PA: 76% SA: 54% PU: 42% ILO: 28%) and use as bait of small cetaceans (PA: 85% SA: 54% PU: 33% ILO: 26%) are now less common than when they started fishing.
Knowledge about Peruvian protective legislation for dolphins among the fishers was high (>66% in each port). However, in terms of how well-implemented and respected the law was, a majority of respondents from Salaverry indicated that the ban was not enforced (54%), while the majority of respondents from the remaining ports (PA: 29% PU: 58% ILO: 48%) indicated that the law was followed.
Factors Affecting the Use of Dolphins as Bait
A total of 112 data points were used in the GLM. The best fitting model for explaining the use of dolphins as bait as identified by minimum AIC included the covariates bycatch, port and gear (Table 3). The significant covariate bycatch indicated that those fishers who used dolphins as bait had a higher occurrence of dolphin bycatch (with a higher probability prediction, Table 4). With regards to the covariate port, our model showed that Salaverry had the highest occurrence of dolphin use as bait while Ilo had the lowest. Fishers who used gillnets used dolphins as bait more frequently than fishers with longlines. Fishers from Salaverry that use gillnets and have dolphin bycatch have the highest probability of using dolphins as bait (Table 3).
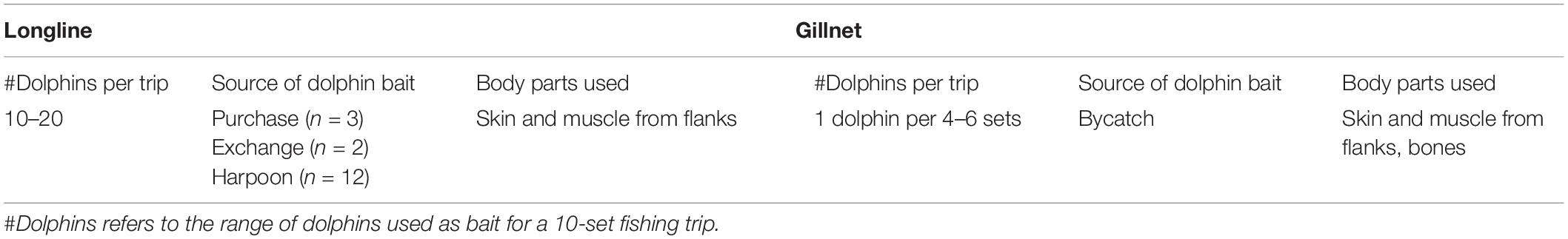
Table 4. Summary results of in-depth interviews with longline and gillnet fishers (n = 12) in Salaverry and Pucusana.
In-Depth Individual Interviews
We interviewed 12 fishers from two ports [Salaverry: 6 (gillnets: 4 and longline: 2), Pucusana: (gillnet: 3 and longline: 3)] to gather more detailed information about the illegal use of dolphins and their use as bait. We also explored the principal reasons why fishers use dolphins.
Availability and Illegal Commerce of Dolphins
Longline fishers from both ports (n = 5) indicated that small cetacean bycatch is rare during longline fishing trips (targeting mako Isurus oxyrinchus and blue sharks Prionace glauca). Fishers commented that they always use fresh bait on all sets. Sometimes they buy fish bait in the port, such as mackerel to use by itself or mix with dolphin bait. This would cost between 900 and 1500 USD for a 10-set trip. However, it is more common that fishers target dolphins (with harpoons) to use them as bait. Longline fishers from Pucusana indicated that they bought or traded dolphins from gillnet fishers at sea for 30 USD per dolphin. Similarly, in Salaverry the estimated cost was 15 USD per dolphin. This sale can be done at sea or, less frequently, at specific locations on the beach. Fishers from Salaverry also reported obtaining dolphin bait by searching at sea for approximately 4–7 days before their fishing activity, investing approximately 600 USD in fuel and food. However, fishers did also mention that these hunts were a practice that is diminishing with time and that it is now harder to find dolphins. Gillnet fishers interviewed mentioned that they do not buy bait, and only use dolphins from bycatch events.
Characteristics of Dolphin Use
Longlines
Longline fishers from Pucusana explained that they use at least two dolphins per set and approximately 10–20 dolphins for a 10-set trip (Table 4). Longlines fishers from Salaverry responded similarly, explaining that around 15–20 dolphins are used for a 15-day fishing trip with 1,000–2,000 hooks (1 dolphin per day). The most commonly used species were dusky and common dolphins. Fishers reported using the skin, blubber and muscle from the side flanks of the dolphin (Table 4), cut into cubes and attached to each or spaced hooks, depending on the availability. Considering only one fishing trip a month per vessel, for a combined fleet of 23 vessels for Pucusana and Salaverry (Alfaro-Shigueto et al., 2010), during the shark fishery season (June–November), a conservative estimate would be in the mid-thousands of dolphins used annually for these two ports.
Gillnets
In gillnets, fishers use the same dolphin parts as longlines, cut into cubes and attached to the net at spaced intervals. One of the interviewed fishers reported that in some cases they even hang the bones from the net. Interviewed fishers from Salaverry noted having about 2–4 dolphins per fishing trip as bycatch. Of these, two dolphins would serve as bait for a whole trip (1 dolphin for 4–6 sets). The other dolphins are usually discarded, or, very rarely, used for food on the boat. One gillnet fisher commented that they will harpoon only one dolphin at the beginning of the fishing trip, as they would likely have a cetacean bycatch in the following sets that could be used as bait. Interviewed gillnet fishers confirmed that dolphins are used as bait when the target fishery is mako and blue sharks. They do not consider small cetaceans as an effective bait for hammerhead sharks (Sphyrna zygaena), which is another common shark fishery in these ports.
Drivers of Use of Bait
When asked why dolphins were preferred as bait, fishers stated that it was a combination of bait effectiveness, availability, and cost. Fishers indicated that dolphin meat is “greasy” and “releases a lot of blood,” making it effective as bait. Another preferred bait was mackerel, but this species is not always available, and its price varies (i.e., availability, if fresh or frozen). Buying alternative bait before fishing trips has associated costs as well, such as ice and transport. Fishers mentioned alternatives baits such as mullet (Mugil cephalus), “tamborin” and Pacific squid although the latter is more commonly used in the dolphinfish fishery.
Fishers also mentioned that using dolphins as bait was less expensive than buying traditional fish bait. Earnings of a given trip needed to be at least 4500–6250 USD (15,000–20,000 PEN) to be profitable and if the price of mako or blue sharks is low, they must catch even higher quantities of sharks to earn a profit. A fisher from Pucusana commented, “We use dolphin bait on every fishing trip, but we don’t do as many trips as we used to before, we lose money.” Fishers mentioned that the use of dolphins as bait could be reduced if the government subsidized baits, or the price of shark products increased at the port.
Discussion
Responses from the surveys and the interview and results from the model were broadly consistent while interviews also allowed us to explore additional topics in greater depth. Our results show that the use of dolphins as bait remains prevalent in Peruvian shark fisheries despite national legislation banning the capture and commerce of marine mammals. In the ports of Pucusana and Salaverry this practice has occurred since at least the 1990s (Van Waerebeek and Reyes, 1994; Mangel et al., 2010) in both the gillnet and longline shark fisheries. Similar to other studies, our results show that gillnets have higher rates of bycatch of small cetaceans that then lead to the use of dolphins as bait on both longline and gillnet vessels. Our results also show the use of dolphins as bait in longline fisheries from the very north and the very south of the country. We think that it is likely therefore that usage rates and practices are similar for longline vessels targeting sharks operating from the other ports along the coast (e.g., Callao, Chimbote). It is also noteworthy that during the early 1990s, to reduce fisheries targeting dolphins, the use of longline gear was reintroduced in Peru as an alternative (and more selective) fishing gear (Reyes, 1993). This fishery is now widespread along Peru’s coast. Unfortunately, as this and previous studies have shown, longlines have in fact introduced a new risk for cetaceans through the use of dolphins as longline bait (Van Waerebeek et al., 1999; Mangel et al., 2010).
Bycatch was reported by most of the fishers, more so in gillnets for both the surveys (SA:79%, PU: 75%) and interview results. Although the majority reported discarding small cetaceans after a bycatch event, a portion of fishers reported using individuals for bait (PA: 27%, SA: 17%, PU: 5%, ILO: 9%). This is similar to our results from interviews, as gillnet fishers reported using bycatch as bait in subsequent sets of their fishing trips. Variations in the frequency of reports of the use of dolphin as bait between surveys and interviews could be due to the illegal nature of the practice, and fear of legal retaliation. Under-reporting and non-response bias related to illegal activities have been suggested by other studies that use surveys (Tourangeau and Yan, 2007; Dewhurst-Richman et al., 2019). We could not calculate the relative biases resulting from these different factors, so true relative mortality levels associated with both bycatch and targeted catch could differ from the results of our survey data. Nevertheless, by combining surveys with in-depth personal interviews, we were able to confirm that the preferred bait for shark fisheries in Peru are dolphins and that fishers rate dusky and common dolphins as the most effective bait.
Regional Context
The use of dolphins as bait has been reported in other countries in Latin America, such as Ecuador (Castro et al., 2020), Colombia (Avila et al., 2008), Chile (Lescrauwaet and Gibbons, 1994), and Argentina (Goodall and Schiavini, 1994). With our statistical analysis we hoped to elucidate associations between variables such as use as bait to port location or type of fishery. As our sample size was limited by strata, however, our statistical results are better interpreted as probable relationships between variables. Our results indicate that the use of dolphins as bait is more prevalent as one moves northward along the Peru coast. But our results also show that this practice likely occurs, to varying degrees, along the entire coast. Similarly, Mintzer et al. (2018) noted that the use of dolphins as bait seems to be more common in the northern countries of Pacific South America (and into Central America), which is possibly related to the high demand for shark meat and products in these countries (Barbosa-Filho et al., 2016; Mintzer et al., 2018).
Reducing Use as Bait
We identified three drivers for the use of dolphins as bait: effectiveness, availability and cost. This was a result of both the interviews and surveys, as they were complementary. This powerful combination of factors is difficult to overcome, although we consider that better management of the shark fisheries could benefit not only cetaceans but also the target species, including blue sharks and shortfin mako sharks which are classified by IUCN as Near Threatened and Vulnerable, respectively (Rigby et al., 2019a,b). Its strong odor, slower disintegration, and hardy structure have made dolphin meat and blubber a preferred bait for shark fisheries (Dayaratne and Joseph, 1993; Mangel et al., 2010; Mintzer et al., 2018). This extends to freshwater habitats, piracatinga fishers believe that the Amazon river dolphins’ smell and blubber strength allows higher productivity than alternative baits (Iriarte and Marmontel, 2014). As stated in the in-depth personal interviews, harpooning dolphins to use as bait in longline fisheries has decreased during the last two decades. This could be attributable to smaller dolphin populations or to an increase in awareness in fisher communities. It also provides an opportunity to motivate fishers into changing this practice. Reversing or changing this behavior will likely require well-thought out socio-economic programs that consider working with fishers at all levels (e.g., community-based programs, co-management schemes).
Recent market regulations by the United States requiring nations exporting fish products to the United States to have similar marine mammal protection measures as those specified in the Marine Mammal Protection Act, might also act as a market regulator of the shark fisheries (Williams et al., 2016). Cases in which the use of marine mammals as bait was mitigated were by implementing multiple strategies atthesametime. For example, the Chilean crab fishery successfully reduced the use of cetaceans by reorienting fisheries from crabs to sea urchins, providing alternative bait, and adjusting to international regulations along with education campaigns and better law enforcement (Young and Iudicello, 2007).
Costing an Illegal Practice
During this study, longline fishers mentioned investing about 600 USD per fishing trip while searching for dolphins for use as bait. This is still cheaper than buying traditional baits for one fishing trip. However, this comparison and its impact on the decision making of fishers requires further development and research because fishers currently do not seem to fully factor their time, risk and labor into the costs associated with an illegal practice. Moreover, if dolphins continue to become scarcer in these fishing zones, it may eventually become more cost effective to use traditional bait or identify new options.
Knowledge Gaps
Important knowledge gaps were identified during our research. First, information that disaggregates deliberate dolphin takes from incidental catch is necessary. Many of the fishers we interviewed reported using bycaught individuals that were traded at sea. This was also mentioned by Avila et al. (2018) – areas where bycatch and harvesting occur were complementary when they reviewed global risks for marine mammals. To better understand the effect this practice has, we must be able to separate how many individuals from each species are caught from targeting or incidental catch and apply mitigation measures accordingly. Implementing bycatch reduction technologies such as pingers or LED lights could reduce the availability of small cetaceans and thus reduce its use as bait (Mangel et al., 2013; Bielli et al., 2020).
Also, in the Eastern Pacific, most small cetacean species lack population estimates, making it difficult to assess the impact this practice has on these populations (Rosa et al., 2012). However, two of the frequently bycaught species are already considered threatened, the Burmeister’s porpoise and the dusky dolphin are listed, respectively, as Endangered and Vulnerable by the IUCN. The latter was mentioned both in surveys and in personal interviews as one of the preferred species to use as bait. Finally, a comparison between alternative baits should be further researched. Fishers have been using dolphin bait for at least the past 20 years and fuel and food prices have varied during that time. Using dolphins as bait could now be more of an ingrained practice rather than a conscious decision. There may now be an opportunity to reduce the use of dolphins as bait and conserve small cetacean populations in the southeastern Pacific Ocean while promoting a more sustainable shark fishery.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the University of Exeter Ethics Committee (2016/1432). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
AP-P and EC: fieldwork and data analysis. AP-P, EC, JM, and JA-S: study design and writing the article. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the WWF Peru Grant Nos. GD30, GD93, and GZ67.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We want to thank the fishers that participated in the surveys and interviews in the sampled ports and the ProDelphinus staff that helped collect field data. We also want to acknowledge WWF Peru who supported the field work of the study through a grant from Ocean Care. We thank reviewers and editor for their valuable comments that improved the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.534507/full#supplementary-material
References
Alfaro-Shigueto, J., Mangel, J. C., Pajuelo, M., Dutton, P. H., Seminoff, J. A., Godley, B. J., et al. (2010). Where small can have a large impact: structure and characterization of small-scale fisheries in Peru. Fish. Res. 106, 8–17. doi: 10.1016/j.fishres.2010.06.004
Antunes Zappes, C., da Silva, C. V., Pontalti, M., Lauriano Danielski, M., and Di Beneditto, A. P. M. (2013). The conflict between the southern right whale and coastal fisheries on the Southern coast of Brazil. Mar. Policy 38, 428–437. doi: 10.1016/j.marpol.2012.07.003
Avila, I. C., Garcia, C., and Bastidas, J. C. (2008). A note on the use of Dolphins as bait in the artisanal fisheries off Bahía Solano, Chocó, Colombia. J. Cetacean Res. Manag. 10, 179–182.
Avila, I. C., Kaschner, K., and Dormann, C. F. (2018). Current global risks to marine mammals: taking stock of the threats. Biol. Conserv. 221, 44–58. doi: 10.1016/j.biocon.2018.02.021
Barbosa-Filho, M. L. V. V., Costa-Neto, E. M., and Danilewicz, D. (2016). Dolphin harpooning off the coast of Bahia, Brazil. Mar. Biodivers. Rec. 9, 1–3. doi: 10.1186/s41200-016-0046-1
Bielli, A., Alfaro-Shigueto, J., Doherty, P. D., Godley, B. J., Ortiz, C., Pasara, A., et al. (2020). An illuminating idea to reduce bycatch in the Peruvian small-scale gillnet fishery. Biol. Conserv. 241:108277. doi: 10.1016/j.biocon.2019.108277
Biernacki, P., and Waldorf, D. (1981). Snowball sampling: problems and techniques of chain referral sampling. Sociol. Methods Res. 10, 141–163. doi: 10.1177/004912418101000205
Braulik, G. T., Kasuga, M., Wittich, A., Kiszka, J. J., Maccaulay, J., Gillespie, D., et al. (2018). Cetacean rapid assessment: an approach to fill knowledge gaps and target conservation across large data deficient areas. Aquat. Conserv. Mar. Freshw. Ecosyst. 28, 216–230. doi: 10.1002/aqc.2833
Brum, S., da Silva, V. M. F., Rossoni, F., and Castilla, J. C. (2015). Use of dolphins and caimans as bait for Calophysus macropterus (Lichtenstein, 1819) (Siluriforme: Pimelodidae) in the Amazon. J. Appl. Ichthyol. 31, 675–680. doi: 10.1111/jai.12772
Burnham, K. P., and Anderson, D. R. (eds) (2004). Model Selection and Multimodel Inference. New York, NY: Springer. doi: 10.1007/b97636
Campbell, E., Mangel, J. C., Alfaro-Shigueto, J., Mena, J. L., Thurstan, R. H., and Godley, B. J. (2020). Coexisting in the Peruvian Amazon: interactions between fisheries and river dolphins. J. Nat. Conserv. 56:125859. doi: 10.1016/j.jnc.2020.125859
Castro, C., Van Waerebeek, K., Cárdenas, D., and Alava, J. (2020). Marine mammals used as bait for improvised fish aggregating devices in marine waters of Ecuador, Eastern tropical Pacific. Endanger. Species Res. 41, 289–302. doi: 10.3354/esr01015
Cegarra, A. M. G., and Pacheco, A. S. (2017). Whale−watching trips in Peru lead to increases in tourist knowledge, pro−conservation intentions and tourist concern for the impacts of whale−watching on humpback whales. Aquat. Conserv. Mar. Freshw. Ecosyst 21, 1011–1020. doi: 10.1002/aqc.2754
Clarke, R. (1962). Whale observation and whale marking off the coast of Chile in 1958 and from Ecuador towards and beyond the Galápagos Islands in 1959. Nor. Hvalfangst Tidende 51, 265–287.
Cosentino, A. M., and Fisher, S. (2016). The utilization of aquatic bushmeat from small cetaceans and manatees in South America and West Africa. Front. Mar. Sci. 3:163. doi: 10.3389/fmars.2016.00163
Davidson, A. D., Boyer, A. G., Kim, H., Pompa-Mansilla, S., Hamilton, M. J., Costa, D. P., et al. (2012). Drivers and hotspots of extinction risk in marine mammals. Proc. Natl. Acad. Sci. U.S.A. 109, 3395–3400. doi: 10.1073/pnas.1121469109
Dawson, S., Slooten, E., DuFresne, S., Wade, P., and Clement, D. (2004). Small-boat surveys for coastal dolphins: line-transect surveys for Hector’s dolphins (Cephalorhynchus hectori). Fish. Bull. 102, 441–451.
Dayaratne, P., and Joseph, L. (1993). A Study on Dolphin Catches in Sri Lanka. Bay of Bengal Programme. Madras, India. Available online at: http://www.fao.org/3/AD883E/AD883E00.htm (accessed May 6, 2020).
Dewhurst-Richman, N. I., Jones, J. P. G. G., Northridge, S., Ahmed, B., Brook, S., Freeman, R., et al. (2019). Fishing for the facts: river dolphin bycatch in a small-scale freshwater fishery in Bangladesh. Anim. Conserv 23, 160–170. doi: 10.1111/acv.12523
Doherty, P. D., Alfaro-Shigueto, J., Hodgson, D. J., Mangel, J. C., Witt, M. J., Godley, B. J., et al. (2014). Big catch, little sharks: insight into Peruvian small-scale longline fisheries. Ecol. Evol. 4, 2375–2383. doi: 10.1002/ece3.1104
Dowling, R. K. (1993). Tourism planning, people and the environment in Western Australia. J. Travel Res. 31, 52–58. doi: 10.1177/004728759303100408
Gonzalez-Pestana, A., Kouri, J. C., and Velez-Zuazo, X. (2016). Shark fisheries in the Southeast Pacific: a 61-year analysis from Peru [version 2; referees: 1 approved, 2 approved with reservations]. F1000Res 3:164. doi: 10.12688/F1000RESEARCH.4412.2
Goodall, R. N. P., and Schiavini, A. C. M. (1994). Net Fisheries and Net Mortality of Small Cetaceans off Tierra del Fuego, Argentina. Report of the International Whaling Commission, 295–304.
Iriarte, V., and Marmontel, M. (2014). Insights on the use of dolphins (boto, Inia geoffrensis and tucuxi, Sotalia fluviatilis) for bait in the piracatinga (Calophysus macropterus) fishery in the western Brazilian Amazon. J. Cetacean Res. Manag. 13, 163–173.
Lescrauwaet, A., and Gibbons, J. (1994). Mortality of small cetaceans and the crab bait fishery in the Magallanes area of Chile since 1980. Rep. Int. Whal. Comm. 2, 485–494.
Mangel, J. C., Alfaro Shigueto, J., Witt, M. J., Hodgson, D. J., and Godley, B. J. (2013). Using pingers to reduce bycatch of small cetaceans in Peru’s small-scale driftnet fishery. Oryx 47, 595–606. doi: 10.1017/S0030605312000658
Mangel, J. C., Alfaro-Shigueto, J., Van Waerebeek, K., Cáceres, C., Bearhop, S., Witt, M. J., et al. (2010). Small cetacean captures in Peruvian artisanal fisheries: high despite protective legislation. Biol. Conserv. 143, 136–143. doi: 10.1016/j.biocon.2009.09.017
Mintzer, V. J., Diniz, K., and Frazer, T. K. (2018). The use of aquatic mammals for bait in global fisheries. Front. Mar. Sci. 5:191. doi: 10.3389/fmars.2018.00191
Mintzer, V. J., Martin, A. R., da Silva, V. M. F., Barbour, A. B., Lorenzen, K., and Frazer, T. K. (2013). Effect of illegal harvest on apparent survival of Amazon river dolphins (Inia geoffrensis). Biol. Conserv. 158, 280–286. doi: 10.1016/j.biocon.2012.10.006
Moore, J. E., Cox, T. M., Lewison, R. L., Read, A. J., Bjorkland, R., McDonald, S. L., et al. (2010). An interview-based approach to assess marine mammal and sea turtle captures in artisanal fisheries. Biol. Conserv. 143, 795–805. doi: 10.1016/j.biocon.2009.12.023
Poonian, C. N. S. S., Hauzer, M. D., Ben Allaoui, A., Cox, T. M., Moores, J. E., Read, A. J. A., et al. (2008). Rapid assessment of sea turtle and marine mammal bycatch in the Union of the Comoros. West. Indian Ocean J. Mar. Sci. 7, 207–215. doi: 10.4314/wiojms.v7i2.48279
Quintana-Rizzo, E. (2014). Harpooning and entanglement of wild dolphins in the Pacific coast of Guatemala. Lat. Am. J. Aquat. Mamm. 9, 179–182.
R Core Team (2017). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Read, A. J. (2008). The looming crisis: interactions between marine mammals and fisheries. J. Mammal. 89, 541–548. doi: 10.1644/07-mamm-s-315r1.1
Read, A. J., Drinker, P., and Northridge, S. (2006). Bycatch of marine mammals in U.S. and global fisheries. Conserv. Biol. 20, 163–169. doi: 10.1111/j.1523-1739.2006.00338.x
Read, A. J., Van Waerebeek, K., Reyes, J. C., McKinnon, J. S., and Lehman, L. C. (1988). The exploitation of small cetaceans in Coastal Peru. Biol. Conserv. 46, 53–70. doi: 10.1016/0006-3207(88)90108-5
Reeves, R. R., McClellan, K., and Werner, T. B. (2013). Marine mammal bycatch in gillnet and other entangling net fisheries, 1990 to 2011. Endanger. Species Res. 20, 71–97. doi: 10.3354/esr00481
Reyes, J. C. (1993). Re-Introduction of Longlines in the Peruvian Shark Fishery: An Alternative to Reduce Small Cetacean Mortality. Cetacean Specialist Group, Species Survival Commission of the International Union for the Conservation of Nature and Natural Resources (IUCN), Gland, Switzerland, and Whale and Dolphin Conservation Society, Wiltshire, UK.
Richards, S. A. (2008). Dealing with overdispersed count data in applied ecology. J. Appl. Ecol. 45, 218–227. doi: 10.1111/j.1365-2664.2007.01377.x
Rigby, C. L., Barreto, R., Carlson, J., Fernando, D., Fordham, S., Francis, M. P., et al. (2019a). Isurus oxyrinchus. IUCN Red List Threat. Species 2019 doi: 10.2305/IUCN.UK.2019-1.RLTS.T39341A2903170.en
Rigby, C. L., Barreto, R., Carlson, J., Fernando, D., Fordham, S., Francis, M. P., et al. (2019b). Prionace glauca. IUCN Red List Threat. Species 2019 e.T39381A2915850, Vol. 59. 355–356. doi: 10.1134/s0042875219030056
Rosa, G. A., Zappes, C. A., and Di Beneditto, A. P. M. (2012). Etnoecologia de pequenos cetáceos: interações entre a pesca artesanal e golfinhos no norte do estado do Rio de Janeiro, Brasil. Biotemas 25, 293–304. doi: 10.5007/2175-7925.2012v25n3p293
Ross, G. J. B. (2006). Review of the Conservation Status of Australia’ s Smaller Whales and Dolphins. Available online at: http://www.environment.gov.au/system/files/resources/8b0f90f9-9834-468a-890b-2c4847a1a107/files/conservation-smaller-whales-dolphins.pdf (accessd October 11, 2019).
Scott, N. J., and Parsons, E. C. M. (2005). A survey of public opinion in south-west Scotland on cetacean conservation issues. Aquat. Conserv. Mar. Freshw. Ecosyst. 15, 299–312. doi: 10.1002/aqc.662
Tourangeau, R., and Yan, T. (2007). Sensitive questions in surveys. Psychol. Bull. 133, 859–883. doi: 10.1037/0033-2909.133.5.859
Van Waerebeek, K., and Reyes, J. C. (1990). Catch of small cetaceans at Pucusana Port, central Peru, during 1987. Biol. Conserv. 51, 15–22. doi: 10.1016/0006-3207(90)90028-N
Van Waerebeek, K., and Reyes, J. C. (1994). “Post-ban small cetacean takes off Peru: a review,” in Gillnets and Cetaceans, eds W. F. Perrin, G. P. Donovan, and J. Barlow (Cambridge: International Whaling Commission), 503–519.
Van Waerebeek, K., Van Bressem, M. F., Alfaro Shigueto, J., Sanino, G. P., Montes, D., and Ontón, K. (1999). “A preliminary analysis of recent captures of small cetaceans in Peru and Chile,” in Proceedings of the 51st Annual Meeting of the International Whaling Commission Scientific Committee, Grenada, 17.
Walpole, M. J., and Goodwin, H. J. (2010). Local attitudes towards conservation and tourism around Komodo National Park, Indonesia. Environ. Conserv. 28, 160–166. doi: 10.1017/S0376892901000169
Williams, R., Burgess, M. G., Ashe, E., Gaines, S. D., Randall, R., Williams, R., et al. (2016). Marine mammal protections require increased global capacity. Science 354, 1372–1374.
Keywords: aquatic wild meat, illegal bait, small cetaceans, shark fisheries, Peru
Citation: Campbell E, Pasara-Polack A, Mangel JC and Alfaro-Shigueto J (2020) Use of Small Cetaceans as Bait in Small-Scale Fisheries in Peru. Front. Mar. Sci. 7:534507. doi: 10.3389/fmars.2020.534507
Received: 12 February 2020; Accepted: 04 September 2020;
Published: 08 October 2020.
Edited by:
Jeremy Kiszka, Florida International University, United StatesReviewed by:
Cornelia Oedekoven, University of St Andrews, United KingdomEric Gilman, The Safina Center, United States
Copyright © 2020 Campbell, Pasara-Polack, Mangel and Alfaro-Shigueto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joanna Alfaro-Shigueto, jalfaros@ucientifica.edu.pe
†These authors have contributed equally to this work
 Elizabeth Campbell
Elizabeth Campbell Andrea Pasara-Polack
Andrea Pasara-Polack Jeffrey C. Mangel
Jeffrey C. Mangel Joanna Alfaro-Shigueto
Joanna Alfaro-Shigueto