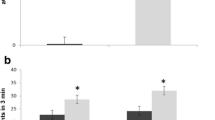
We examined the content of autoantibodies against brain proteins, content of myelin basic protein (MBP), and level of NO synthesis in the cerebellum and cerebral hemispheres on day 18 of pregnancy in BALB/c mice with the experimental antiphospholipid syndrome (APS); the effects of L-arginine on the above indices were also evaluated. As was found, the contents of autoantibodies against brain proteins having the molecular masses 120, 150, and > 170 kDa were greater than in the control. Under APS conditions, the amount of eNOS-produced NO was relatively insufficient; this was observed against the background of total hyperproduction of NO synthesized by iNOS in blood serum. In APS mice, the contents of stable NO metabolites, NO2– and NO3–, in the cerebellum were higher, while these levels in the cerebral hemispheres were lower with respect to the control. There were reasons to believe that the effects of L-arginine under APS conditions of and cerebral dysfunction are provided not only at the expense of influences upon the NO system, but also via antioxidant and cytoprotective properties of this amino acid. In pregnant APS mice, the content of MBP (95–110 kDa) in the cerebellum and that of MBP (18.4 kDa) in the cerebral hemispheres were greater than in the control. In APS animals, administration of L-arginine provided increase in the content of MBP (18.4 kDa) in the cerebral hemispheres compared with the respective index with no treatment. Our results show that the remyelination processes in animals with the APS are activated; this may be interpreted as a compensatory response to the injury.
Similar content being viewed by others
References
T. Fleetwood, R. Cantello, and C. Comi, “Antiphospholipid syndrome and the neurologist: from pathogenesis to therapy,” Front. Neurol., 9, 1001 doi:https://doi.org/10.3389/fneur.2018.01001 (2018).
L. A. Kalashnikova, “Cerebrovascular disorders in antiphospholipid syndrome,” Ann. Klin. Eksper. Nevrolog., 5, No. 1, 39–43 (2011).
N. Costedoat-Chalumeau, G. Guettrot-Imbert, and V. Leguern, “Pregnancy and antiphospholipid syndrome,” Rev. Med. Interne., 33, No. 4, 209–216 (2012).
E. L. Nasonov, Antiphospholipid Syndrome, Litterra, Moscow (2004).
A. B. Poletayev and F. Aliyeva, “Autoantibodies and the immunopathology of pregnancy,” Prakt. Med., No. 43, 20–24 (2010).
V. A. Sobolyev, V. A. Proshyn, S. G. Morozov, et al., “State of health of children born by mothers with different contents of antibodies against a few nerve tissue proteins and protein fractions,” Pediatriya, 5, 44–50 (2004).
I. F. Ricarte, L. A. Dutra, F. F. Abrantes, et al., “Neurologic manifestations of antiphospholipid syndrome,” Lupus, 27, No. 9, 1404–1414, https://doi.org/10.1177/0961203318776110(2018).
J. Graf, “Central nervous system manifestations of antiphospholipid syndrome,” Rheum. Dis. Clin. North. Am., 43, No. 4, 547–560 (2017).
T. G. D’Aversa, E. A. Eugenin, L. Lopez, et al., “Myelin basic protein induces inflammatory mediators from primary human endothelial cells and blood-brain barrier disruption: implications for the pathogenesis of multiple sclerosis,” Neuropathol. Appl. Neurobiol., 39, No. 3, 270–283 (2013).
A. V. Astakhin, O. O. Yevlasheva, and B. N. Levitan, “Clinical and diagnostic importance of the myelin basic protein and neurospecific enolase in medical practice,” Astrakhan. Med. J., 4, No. 11, 9–17 (2016).
P. R. J. Ames, J. R. Batuca, A. Ciampa, et al., “Clinical relevance of nitric oxide metabolites and nitrative stress in thrombotic primary antiphospholipid syndrome,” J. Rheumatol., 37, No. 12, 2523–2530 (2010).
V. P. Reutov, E. G. Sorokina, N. V. Samosudova, et al., “Geodynamics of the brain: Glutamatergic system and nitric oxide cycle in the regulation of cerebral circulation. A novel concept,” Pacific Med. J., 3, 37–45 (2017).
Yu. M. Stepanov, I. N. Kononov, A. I. Zhurbina, et al., “Arginine in medical practice (a review),” Zh. AMS Ukraine, 10, No. 1, 340–352 (2004).
G. V. Zaichenko, Yu. B. Lar’yanovs’ka, and T. V. Deyeva, “Morphological state of the womb and placenta in expetimental modeling of the gestation antiphospholipid syndrome in mice,” Ukr. Med. Almanakh, 14, No. 4, 136–141 (2011).
О. H. Lowry, N. J. Rosebrough, A. L. Farr, and R. J. Randall, “Protein measurement with the Folin phenol reagent,” J. Biol. Chem., 193, No. 1, 265–275 (1951).
U. K. Laemmli, “Cleavage of structural proteins during the assembly of the head of bacteriophage T4,” Nature, 227, No. 5259, 680–685 (1970).
H. Towbin, T. Staehelin, and J. Gordon, “Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications”, Proc. Natl. Acad. Sci. USA, 76, No. 9, 4350–4354 (1979).
L. C. Green, D. A. Wagner, J. Glogowski, et al., “Analysis of nitrate, nitrite and [15N] nitrate in biological fluids,” Analyt. Biochem., 126, No. 1, 131–138 (1982).
I. O. Kiselyk, M. D. Lutsyk, and L. Yu. Shevchenko, “Peculiarities of estimation of the nitrate and nitrite amounts in blood of the patients with virus hepatitis and jaundices related to other pathologies,” Lab. Diagnostics, 3, 43–45 (2001).
A. B. Poletaev and S. G. Morozov, “Changes of maternal serum natural antibodies of IgG class to proteins МBP, S100, ACBP14/18 and MP65 and embryonicmisdevelopments in humans,” Human Antibody, 9, No. 4, 216–222 (2000).
V. A. Aleinik, S. M. Babich, Kh. N. Negmatasheva, et al., “Peculiarities of the immunological shifts in women with miscarriage and the presence of autoantibodies,” Molod. Uchen., No. 22, 411–413 (2017).
N. Bizzaro, “Autoantibodies as predictor of disease: the clinical and experimental evidence”, Autoimmun. Rev., 6, No. 6, 325–333 (2007).
J. D. Alves, L. J. Mason, P. R. J. Ames, et al., “Antiphospholipid antibodies are associated with enhanced oxidative stress, decreased plasma nitric oxide and paraoxonase activity in an experimental mouse model,” Rheumatology (Oxford), 44, No. 10, 1238–1244 (2005).
S. Ramesh, C. N. Morrell, C. Tarango, et al., “Antiphospholipid antibodies promote leukocyte-endothelial cell adhesion and thrombosis in mice by antagonizing eNOS via β2GPI and apoER2,” J. Clin. Invest., 121, No. 1, 120–131 (2011).
C. Mineo, “Inhibition of nitric oxide and antiphospholipid antibody-mediated thrombosis,” Curr. Rheumatol. Rep., 15, No. 5, 324 (2013).
E. Svenungsson, M. Andersson, L. Brundin, et al., “Increased levels of proinflammatory cytokines and nitric oxide metabolites in neuropsychiatric lupus erythematosus,” Ann. Rheum. Dis., 60, No. 4, 372–379 (2001).
V. O. Kurovs’ka, V. P. Pishak, and S. S. Tkachuk, “Role of nitric oxide in ischemic and ischemico-reperfusional damages of the brain,” Bukovin. Med. Visn., 12, No. 4, 143–149 (2008).
Ch. López-Pedrera, N. Barbarroja, Y. Jimenez-Gomez, et al., “Oxidative stress in the pathogenesis of atherothrombosis associated with antiphospholipid syndrome and systemic lupus erythematosus: new therapeutic approaches,” Rheumatology (Oxford), 55, No. 12, 2096–2108 (2016).
P. Patrikios, C. Stadelmann, A. Kutzelnigg, et al., “Remyelination is extensive in a subset of multiple sclerosis patients”, Brain, 129, Pt. 12, 3165–3172 (2006).
F. Ruffini, T. E. Kennedy, and J. P. Antel, “Inflammation and remyelination in the central nervous system: a tale of two systems,” Am. J. Pathol., 164, No. 5, 1519–1522 (2004).
H.F. Kuipers, J. Yoon, J.van Horssen, et al., “Phosphorylation of αB-crystallin supports reactive astrogliosis in demyelination”, Proc. Natl. Acad. Sci. USA. 114, No. 9, 1745–1754 (2017).
A. Fontana, W. Fierz, and H. Wekerle, “Astrocytes present myelin basic protein to encephalitogenic T-cell lines”, Nature, 307, No. 5948, 273–276 (1984).
H. Kıray, S. L. Lindsay, S. Hosseinzadeh, et al., “The multifaceted role of astrocytes in regulating myelination,” Exp. Neurol., 283, Pt. B, 541–549 (2016).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yaremchuk, O.Z. Contents of Myelin Basic Protein and Autoantibodies against Brain Proteins in the Experimental Antiphospholipid Syndrome. Neurophysiology 52, 116–123 (2020). https://doi.org/10.1007/s11062-020-09860-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11062-020-09860-7