Co-Delivery of Cisplatin and Gemcitabine via Viscous Nanoemulsion for Potential Synergistic Intravesical Chemotherapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Drug-Loaded Formulation Preparation
2.4. Characterization of Microemulsion
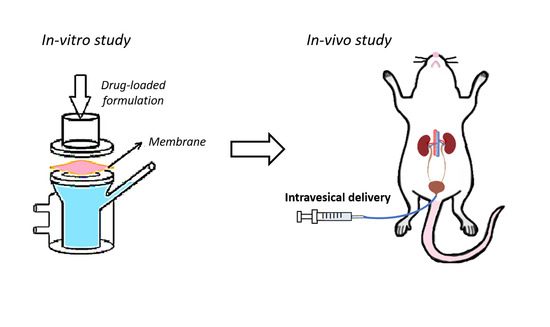
2.5. In Vitro Permeation Studies
2.6. In Vivo Intravesical Administration of Dual-Loaded Formulations
2.7. In Vivo Confocal Laser Scanning Microscopy (CLSM) Analysis
2.8. Irritation Test
2.9. Data Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics
3.2. In Vitro Permeation and Drug Deposition Evaluation
3.3. In Vivo Intravesical Application
3.4. In Vivo CLSM Analysis
3.5. Irritation Test
3.6. Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Miao, L.; Guo, S.; Zhang, Y.; Zhang, L.; Satterlee, A.; Kim, W.Y.; Huang, L. Synergistic anti-tumor effects of combined gemcitabine and cisplatin nanoparticles in a stroma-rich bladder carcinoma model. J. Control. Release: Off. J. Control. Release Soc. 2014, 182, 90–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goel, S.; Sinha, R.J.; Bhaskar, V.; Aeron, R.; Sharma, A.; Singh, V. Role of gemcitabine and cisplatin as neoadjuvant chemotherapy in muscle invasive bladder cancer: Experience over the last decade. Asian J. Urol. 2019, 6, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Waldrop, L. Gemcitabine hydrochloride. Clin. J. Oncol. Nurs. 2001, 5, 77–78. [Google Scholar] [PubMed]
- Bergman, A.M.; Pinedo, H.M.; Peters, G.J. Determinants of resistance to 2′,2′-difluorodeoxycytidine (gemcitabine). Drug Resist. Updates: Rev. Comment. Antimicrob. Anticancer Chemother. 2002, 5, 19–33. [Google Scholar] [CrossRef]
- Chou, R.; Selph, S.; Buckley, D.I.; Fu, R.; Griffin, J.C.; Grusing, S.; Gore, J.L. Intravesical Therapy for the Treatment of Nonmuscle Invasive Bladder Cancer: A Systematic Review and Meta-Analysis. J. Urol. 2017, 197, 1189–1199. [Google Scholar] [CrossRef]
- Kurita, Y.; Yokoyama, A.; Matsui, K.; Hara, N.; Nakai, Y.; Ohhashi, Y.; Niitani, H. Phase I study of gemcitabine hydrochloride (LY 188011) combination therapy with cisplatin in the patients with non-small cell lung cancer. Gan Kagaku Ryoho. Cancer Chemother. 1999, 26, 898–907. [Google Scholar]
- Kunikane, H.; Kurita, Y.; Watanabe, K.; Yokoyama, A.; Noda, K.; Fujita, Y.; Yoneda, S.; Nakai, Y.; Niitani, H. A study of the combination of gemcitabine hydrochloride (LY188011) and cisplatin in non-small-cell lung cancer: 3-week schedule. Int. J. Clin. Oncol. 2001, 6, 284–290. [Google Scholar] [CrossRef]
- Morizane, C.; Okusaka, T.; Mizusawa, J.; Katayama, H.; Ueno, M.; Ikeda, M.; Ozaka, M.; Okano, N.; Sugimori, K.; Fukutomi, A.; et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: The FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1950–1958. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wientjes, M.G.; Dalton, J.T.; Badalament, R.A.; Drago, J.R.; Au, J.L. Bladder wall penetration of intravesical mitomycin C in dogs. Cancer Res. 1991, 51, 4347–4354. [Google Scholar] [PubMed]
- Dalton, J.T.; Wientjes, M.G.; Badalament, R.A.; Drago, J.R.; Au, J.L. Pharmacokinetics of intravesical mitomycin C in superficial bladder cancer patients. Cancer Res. 1991, 51, 5144–5152. [Google Scholar] [PubMed]
- Chai, M.; Wientjes, M.G.; Badalament, R.A.; Burgers, J.K.; Au, J.L. Pharmacokinetics of intravesical doxorubicin in superficial bladder cancer patients. J. Urol. 1994, 152 Pt 1, 374–378. [Google Scholar] [CrossRef]
- Song, D.; Wientjes, M.G.; Au, J.L. Bladder tissue pharmacokinetics of intravesical taxol. Cancer Chemother. Pharmacol. 1997, 40, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Guo, H.; Li, D.; Hou, Y.; Kuang, T.; Ding, J. Intravesical Hydrogels as Drug Reservoirs. Trends Biotechnol. 2020, 38, 579–583. [Google Scholar] [CrossRef]
- Boonme, P.; Kaewbanjong, J.; Amnuaikit, T.; Andreani, T.; Silva, A.M.; Souto, E.B. Microemulsion and Microemulsion-Based Gels for Topical Antifungal Therapy with Phytochemicals. Curr. Pharm. Des. 2016, 22, 4257–4263. [Google Scholar] [CrossRef]
- Das, S.; Desai, J.L.; Thakkar, H.P. Gemcitabine hydrochloride-loaded functionalised carbon nanotubes as potential carriers for tumour targeting. Indian J. Pharm. Sci. 2013, 75, 707–715. [Google Scholar]
- Kupper, S.; Klosowska-Chomiczewska, I.; Szumala, P. Collagen and hyaluronic acid hydrogel in water-in-oil microemulsion delivery systems. Carbohydr. Polym. 2017, 175, 347–354. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Hsieh, Y.H.; Huang, Y.B.; Chang, J.S.; Huang, C.T.; Wu, P.C. Microemulsions for intravesical delivery of gemcitabine. Chem. Pharm. Bull. 2010, 58, 1461–1465. [Google Scholar] [CrossRef] [Green Version]
- Kates, M.; Date, A.; Yoshida, T.; Afzal, U.; Kanvinde, P.; Babu, T.; Sopko, N.A.; Matsui, H.; Hahn, N.M.; McConkey, D.J.; et al. Preclinical Evaluation of Intravesical Cisplatin Nanoparticles for Non-Muscle-Invasive Bladder Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 6592–6601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rappaport, Y.H.; Zisman, A.; Jeshurun-Gutshtat, M.; Gerassi, T.; Hakim, G.; Vinshtok, Y.; Stav, K. Safety and Feasibility of Intravesical Instillation of Botulinum Toxin-A in Hydrogel-based Slow-release Delivery System in Patients with Interstitial Cystitis-Bladder Pain Syndrome: A Pilot Study. Urology 2018, 114, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.Y.; Chang, I.H.; Goo, Y.T.; Kim, C.H.; Kang, T.H.; Kim, S.Y.; Lee, S.J.; Song, S.H.; Whang, Y.M.; Choi, Y.W. Intravesical delivery of rapamycin via folate-modified liposomes dispersed in thermo-reversible hydrogel. Int. J. Nanomed. 2019, 14, 6249–6268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, T.; Wu, J.; Zhao, X.; Lian, H.; Yuan, A.; Tang, X.; Zhao, S.; Guo, H.; Hu, Y. In situ floating hydrogel for intravesical delivery of adriamycin without blocking urinary tract. J. Pharm. Sci. 2014, 103, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Chancellor, M.B.; Li, Z.; De Groat, W.C.; Yoshimura, N.; Fraser, M.O.; Huang, L. Urodynamic and immunohistochemical evaluation of intravesical capsaicin delivery using thermosensitive hydrogel and liposomes. J. Urol. 2004, 171, 483–489. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Z.; Zhang, G.; Lin, F.; Liu, Y.; Zhang, Y.; Feng, J.; Chen, W.; Meng, Q.; Chen, L. AS1411 Aptamer/Hyaluronic Acid-Bifunctionalized Microemulsion Co-Loading Shikonin and Docetaxel for Enhanced Antiglioma Therapy. J. Pharm. Sci. 2019, 108, 3684–3694. [Google Scholar] [CrossRef]
- Shukla, T.; Upmanyu, N.; Agrawal, M.; Saraf, S.; Saraf, S.; Alexander, A. Biomedical applications of microemulsion through dermal and transdermal route. Biomed. Pharmacother. 2018, 108, 1477–1494. [Google Scholar] [CrossRef]
- Lopez-Flores, A.; Jurado, R.; Garcia-Lopez, P. A high-performance liquid chromatographic assay for determination of cisplatin in plasma, cancer cell, and tumor samples. J. Pharmacol. Toxicol. Methods 2005, 52, 366–372. [Google Scholar] [CrossRef]
- Su, R.; Fan, W.; Yu, Q.; Dong, X.; Qi, J.; Zhu, Q.; Zhao, W.; Wu, W.; Chen, Z.; Li, Y.; et al. Size-dependent penetration of nanoemulsions into epidermis and hair follicles: Implications for transdermal delivery and immunization. Oncotarget 2017, 8, 38214–38226. [Google Scholar] [CrossRef] [Green Version]
- Tuan-Mahmood, T.M.; McCrudden, M.T.; Torrisi, B.M.; McAlister, E.; Garland, M.J.; Singh, T.R.; Donnelly, R.F. Microneedles for intradermal and transdermal drug delivery. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2013, 50, 623–637. [Google Scholar] [CrossRef] [Green Version]
- Majumdar, S.; Hippalgaonkar, K.; Repka, M.A. Effect of chitosan, benzalkonium chloride and ethylenediaminetetraacetic acid on permeation of acyclovir across isolated rabbit cornea. Int. J. Pharm. 2008, 348, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Rhee, Y.S.; Choi, J.G.; Park, E.S.; Chi, S.C. Transdermal delivery of ketoprofen using microemulsions. Int. J. Pharm. 2001, 228, 161–170. [Google Scholar] [CrossRef]
- Huang, C.T.; Tsai, C.H.; Tsou, H.Y.; Huang, Y.B.; Tsai, Y.H.; Wu, P.C. Formulation optimization of transdermal meloxicam potassium-loaded mesomorphic phases containing ethanol, oleic acid and mixture surfactant using the statistical experimental design methodology. J. Microencapsul. 2011, 28, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chang, X.; Weng, T.; Zhao, X.; Gao, Z.; Yang, Y.; Xu, H.; Yang, X. A study of microemulsion systems for transdermal delivery of triptolide. J. Control. Release Off. J. Control. Release Soc. 2004, 98, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Li, Z.; Chancellor, M.; De Groat, W.C.; Yoshimura, N.; Huang, L. Sustained intravesical drug delivery using thermosensitive hydrogel. Pharm. Res. 2004, 21, 832–837. [Google Scholar] [CrossRef]
- Spernath, A.; Aserin, A.; Ziserman, L.; Danino, D.; Garti, N. Phosphatidylcholine embedded microemulsions: Physical properties and improved Caco-2 cell permeability. J. Control. Release Off. J. Control. Release Soc. 2007, 119, 279–290. [Google Scholar] [CrossRef]
- El Maghraby, G.M. Transdermal delivery of hydrocortisone from eucalyptus oil microemulsion: Effects of cosurfactants. Int. J. Pharm. 2008, 355, 285–292. [Google Scholar] [CrossRef]





| Formulation (g) | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| Capyrol | 5.0 | 5.0 | 5.0 | 5.0 |
| Benzalkonium chloride | 0.5 | 1.5 | 2.0 | 2.0 |
| Tween 80 | 5.0 | |||
| 1,5-Pentanediol | 20.0 | 20.0 | 20.0 | 15.0 |
| Carbitol | 10.0 | 10.0 | 10.0 | 10.0 |
| Water | 59.5 | 63.5 | 63.0 | 68.0 |
| Methylcellulose | 2.0 | 2.0 | 2.0 | 2.0 |
| Formulation | Droplet Size nm | PDI | Viscosity Cps |
|---|---|---|---|
| F1 | 10.26 ± 0.15 | 0.06 ± 0.00 | 410.27 ± 1.16 |
| F2 | 12.50 ± 0.10 | 0.14 ± 0.01 | 687.90 ± 3.16 |
| F3 | 14.13 ± 0.42 | 0.16 ± 0.09 | 923.93 ± 1.61 |
| F4 | 26.73 ± 0.06 | 0.30 ± 0.01 | 1104.00 ± 4.70 |
| Gemcitabine | Cisplatin | |||||||
|---|---|---|---|---|---|---|---|---|
| Formulation | FluxG (μg/cm2/h) | ERGF | Deposition (μg/cm2) | ERGD | Flux (μg/cm2/h) | ERCF | Deposition (μg/cm2) | ERCD |
| C | 4.84 ± 0.64 | 1.0 | 0.57 ± 0.24 | 1.0 | 1.30 ± 0.04 | 1.0 | 0.05 ± 0.09 | 1.0 |
| F3 | 92.52 ± 62.93 * | 19.0 | 30.95 ± 7.08 * | 54.0 | 24.67 ± 3.37 * | 18.0 | 0.91 ± 0.30 * | 18.0 |
| F4 | 113.69 ± 27.38 * | 23.5 | 40.36 ± 4.48 * | 70.0 | 17.49 ± 4.09 * | 13.0 | 1.35 ± 0.94 * | 27.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.-Y.; Tsai, M.-J.; Chang, L.-C.; Wu, P.-C. Co-Delivery of Cisplatin and Gemcitabine via Viscous Nanoemulsion for Potential Synergistic Intravesical Chemotherapy. Pharmaceutics 2020, 12, 949. https://doi.org/10.3390/pharmaceutics12100949
Chen T-Y, Tsai M-J, Chang L-C, Wu P-C. Co-Delivery of Cisplatin and Gemcitabine via Viscous Nanoemulsion for Potential Synergistic Intravesical Chemotherapy. Pharmaceutics. 2020; 12(10):949. https://doi.org/10.3390/pharmaceutics12100949
Chicago/Turabian StyleChen, Ting-Yu, Ming-Jun Tsai, Li-Ching Chang, and Pao-Chu Wu. 2020. "Co-Delivery of Cisplatin and Gemcitabine via Viscous Nanoemulsion for Potential Synergistic Intravesical Chemotherapy" Pharmaceutics 12, no. 10: 949. https://doi.org/10.3390/pharmaceutics12100949