Expanding Family of Litharge-Derived Sulfate Minerals and Synthetic Compounds: Preparation and Crystal Structures of [Bi2CuO3]SO4 and [Ln2O2]SO4 (Ln = Dy and Ho)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Single-Crystal XRD Studies
2.3. Powder XRD Studies
3. Results
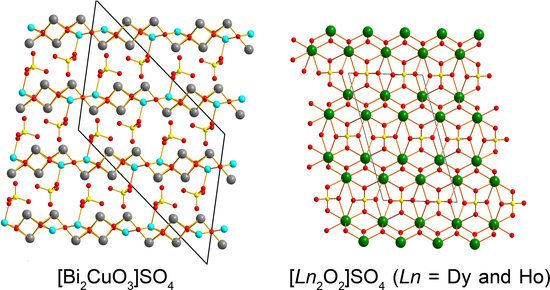
3.1. Сrystal Structure of [Bi2CuO3]SO4
3.2. Сrystal Structure of [Ln2O2]SO4 (Ln = Dy, Ho)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moure, A. Review and perspectives of Aurivillius structures as a lead-free piezoelectric system. Appl. Sci. 2018, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Siidra, O.I.; Krivovichev, S.V.; Filatov, S.K. Minerals and synthetic Pb(II) compounds with oxocentered tetrahedra: Review and classification. Z. Kristallogr. 2008, 223, 114–126. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Mentré, O.; Siidra, O.I.; Colmont, M.; Filatov, S.K. Anion-centered tetrahedra in inorganic compounds. Chem. Rev. 2013, 113, 6459–6535. [Google Scholar] [CrossRef] [PubMed]
- Charkin, D.O. Modular approach as applied to the description, prediction, and targeted synthesis of bismuth oxohalides with layered structures. Russ. J. Inorg. Chem. Suppl. 2008, 53, 1977–1996. [Google Scholar] [CrossRef]
- Cooper, M.; Hawthorne, F.C. The crystal structure of kombatite, Pb14(VO4)2O9Cl4. Am. Miner. 1994, 79, 550–554. [Google Scholar]
- Bonaccorsi, E.; Pasero, M. Crystal structure refinement of sahlinite Pb14(AsO4)2O9Cl4. Mineral. Mag. 2003, 67, 15–21. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Siidra, O.I.; Nazarchuk, E.V.; Burns, P.C.; Depmeier, W. Exceptional topological complexity of lead oxide blocks in Pb31O22X18 (X = Br, Cl). Inorg. Chem. 2006, 45, 3846–3848. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Turner, R.; Rumsey, M.; Siidra, O.I.; Kirk, C.A. The crystal structure of mereheadite. Miner. Mag. 2009, 73, 75–89. [Google Scholar] [CrossRef]
- Leepore, G.O.; Welch, M.D. The crystal structure of parkinsonite, nominally Pb7MoO9Cl2: A naturally occurring Aurivillius phase. Miner. Mag. 2010, 74, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Turner, R.W.; Siidra, O.I.; Krivovichev, S.V.; Stanley, C.J.; Spratt, J. Rumseyite, [Pb2OF]Cl, the first naturally occurring fluoroxychloride mineral with the parent crystal structure for layered lead oxychlorides. Mineral. Mag. 2012, 76, 1247–1255. [Google Scholar] [CrossRef]
- Siidra, O.I.; Krivovichev, S.V.; Turner, R.W.; Rumsey, M.S.; Spratt, J. Crystal chemistry of layered Pb oxychloride minerals with PbO-related structures. Crystal structure of hereroite, [Pb32O20(O,□)](AsO4)2((Si,As,V,Mo)O4)2Cl10. Am. Miner. 2013, 98, 248–255. [Google Scholar] [CrossRef]
- Siidra, O.I.; Krivovichev, S.V.; Turner, R.W.; Rumsey, M.S.; Spratt, J. Crystal chemistry of layered Pb oxychloride minerals with PbO-related structures. II. Crystal structure of vladkrivovichevite, [Pb32O18][Pb4Mn2O]Cl14(BO3)8·2H2O. Am. Miner. 2013, 98, 256–261. [Google Scholar] [CrossRef]
- Siidra, O.I.; Zinyakhina, D.O.; Zadoya, A.I.; Krivovichev, S.V.; Turner, R.W. Synthesis and modular structural architectures of mineralogically inspired novel complex Pb oxyhalides. Inorg. Chem. 2013, 52, 12799–12805. [Google Scholar] [CrossRef] [PubMed]
- Siidra, O.I.; Gogolin, M.; Lukina, E.A.; Kabbour, H.; Bubnova, R.S.; Mentre, O.; Agakhanov, A.A.; Krivovichev, S.V.; Colmont, M.; Gesing, T. Structural evolution from 0D Units to 3D frameworks in Pb oxyhalides: Unexpected strongly corrugated layers in Pb7O6Br2. Inorg. Chem. 2015, 54, 11550–11556. [Google Scholar] [CrossRef] [PubMed]
- Siidra, O.I.; Kabbour, H.; Mentré, O.; Nazarchuk, E.V.; Kegler, P.; Zinyakhina, D.O.; Colmont, M.; Depmeier, W. Lead oxychloride borates obtained under extreme conditions. Inorg. Chem. 2016, 55, 9077–9084. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Nekrasova, D.O.; Siidra, O.I.; Polekhovsky, Y.S.; Pekov, I.V. Janchevite, Pb7V5+(O8.5□0.5)Cl2, a new mineral from Kombat mine, Namibia. Can. Mineral. 2018, 56, 159–165. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Siidra, O.I.; Polekhovsky, Y.S.; Pekov, I.V.; Varlamov, D.A.; Ermolaeva, V.N.; Virus, A.A. Erikjonssonite, (Pb32O21)[(V,Si,Mo,As)O4]4Cl9, a new mineral from the Kombat mine and structural classification of layered lead oxychlorides related to litharge. Eur. J. Miner. 2019, 31, 619–628. [Google Scholar] [CrossRef]
- Perchiazzi, N.; Hålenius, U.; Vignola, P.; Demitri, N. Crystal chemical study of ecdemite from Harstigen, a new natural member of the layered lead oxyhalide group. Eur. J. Miner. 2019, 31, 609–617. [Google Scholar] [CrossRef]
- Bullen, T.D. Stable isotopes of transition and post-transition metals as tracers in environmental studies. In Handbook of Environmental Isotope Geochemistry. Advances in Isotope Geochemistry; Baskaran, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 177–203. [Google Scholar]
- Corkett, A.J.; Chen, Z.; Slabon, A.; Dronskowski, R. Band gap tuning in bismuth oxide carbodiimide Bi2O2NCN. Inorg. Chem. 2019, 58, 6467–6473. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Takahashi, M.; Kikkawa, S.; Kanamaru, F. Synthesis and crystal structure of a new compound, lanthanum dioxymonocyanamide (La2O2CN2). J. Solid State Chem. 1995, 114, 592–594. [Google Scholar] [CrossRef]
- Grice, J.D. A solution to the crystal structures of bismutite and beyerite. Can. Mineral. 2002, 40, 693–698. [Google Scholar] [CrossRef]
- Ziegler, P.; Grigoraviciute, I.; Gibson, K.; Glaser, J.; Kareiva, A.; Meyer, H.J. On the characterization of BiMO2NO3 (M = Pb, Ca, Sr, Ba) materials related with the Sillen X1 structure. J. Solid State Chem. 2004, 177, 3610–3615. [Google Scholar] [CrossRef]
- Charkin, D.O.; Plokhikh, I.V.; Zadoya, A.I.; Kuznetsova, P.L.; Kazakov, S.M.; Siidra, O.I. CdBiO2NO3, a new layered bismuth oxide nitrate. Solid State Sci. 2018, 84, 23–27. [Google Scholar] [CrossRef]
- Huang, H.; Tian, N.; Jin, S.; Zhang, Y.; Wang, S. Syntheses, characterization and nonlinear optical properties of a bismuth subcarbonate Bi2O2CO3. Solid State Sci. 2014, 30, 1–5. [Google Scholar] [CrossRef]
- Hybler, J.; Dušek, M. Revision of the crystal structure of kettnerite CaBi[OFCO3]. Eur. J. Mineral. 2007, 19, 411–418. [Google Scholar] [CrossRef]
- Kampf, A.R. Grandreefite, Pb2F2SO4: Crystal structure and relationship to the lanthanide oxide sulfates, Ln2O2SO4. Am. Mineral. 1991, 76, 278–282. [Google Scholar]
- Kampf, A.R. The crystal structure of Ba2F2(S6+O3S2-), a natural thiosulphate weathering product of old smelting slags at the Surrender Mill, Yorkshire, UK. Mineral. Mag. 2009, 73, 251–255. [Google Scholar] [CrossRef]
- Zhukov, S.G.; Yatsenko, A.; Chernyshev, V.V.; Trunov, V.; Tserkovnaya, E.; Antson, O.; Hölsä, J.; Baules, P.; Schenk, H. Structural study of lanthanum oxysulfate (LaO)2SO4. Mater. Res. Bull. 1997, 32, 43–50. [Google Scholar] [CrossRef]
- Denisenko, Y.G.; Sal’nikova, E.I.; Basova, S.A.; Molokeev, M.S.; Krylov, A.S.; Aleksandrovsky, A.S.; Oreshonkov, A.S.; Atuchin, V.V.; Volkova, S.S.; Khritokhin, N.A.; et al. Synthesis of samarium oxysulfate Sm2O2SO4 in the high-temperature oxidation reaction and its structural, thermal and luminescent properties. Molecules 2020, 25, 1330. [Google Scholar] [CrossRef] [Green Version]
- Hartenbach, I.; Schleid, T. Serendipitous formation of single-crystalline Eu2O2(SO4). Z. Anorg. Allg. Chem. 2002, 628, 2171. [Google Scholar] [CrossRef]
- Charkin, D.O.; Grischenko, R.O.; Sadybekov, A.A.; Goff, R.J.; Lightfoot, P. A new approach to synthesis of layered fluorites containing molecular anions: Synthesis of Ln2O2CO3, K(LnO)CO3, and Ln2O2CrO4 via metathesis reactions. Inorg. Chem. 2008, 47, 3065–3071. [Google Scholar] [CrossRef] [PubMed]
- Charkin, D.O.; Plokhikh, I.V.; Zadoya, A.I.; Zaloga, A.N.; Depmeier, W.; Siidra, O.I. Structural, thermal, and IR studies of β-[Nd2O2](CrO4), an unexpected analog of a slag phase [Ba2F2](S6+O3S2−). Z. Kristallogr. 2019, 234, 1–8. [Google Scholar] [CrossRef]
- Charkin, D.O.; Plokhikh, I.V.; Zadoya, A.I.; Kazakov, S.M.; Zaloga, A.N.; Kozin, M.S.; Depmeier, W.; Siidra, O.I. [Pb2F2](SeO4): A heavier analogue of grandreefite, the first layered fluoride selenate. Phys. Chem. Miner. 2018, 45, 69–76. [Google Scholar] [CrossRef]
- Charkin, D.O.; Karpov, A.S.; Kazakov, S.M.; Plokhikh, I.V.; Zadoya, A.I.; Kuznetsov, A.N.; Maslakov, K.I.; Teterin, A.Y.; Teterin, Y.A.; Zaloga, A.N.; et al. Synthesis, crystal structure, spectroscopic properties, and thermal behavior of rare-earth oxide selenates, Ln2O2SeO4 (Ln = La, Pr, Nd): The new perspectives of solid-state double-exchange synthesis. J. Solid State Chem. 2019, 277, 163–168. [Google Scholar] [CrossRef]
- Charkin, D.O.; Akimov, G.A.; Plokhikh, I.V.; Zaloga, A.N.; Borisov, A.S.; Stefanovich, S.Y.; Kuznetsov, A.N.; Siidra, O.I. Bi2O2SO4, a new representative of the grandreefite structure type. J. Solid State Chem. 2020, 282, 121–124. [Google Scholar] [CrossRef]
- Golovnev, N.N.; Molokeev, M.S.; Vereshchagin, S.N.; Atuchin, V.V. Synthesis and thermal transformation of a neodymium(III) complex [Nd(HTBA)2(C2H3O2)(H2O)2]⋅2H2O to non-centrosymmetric oxosulfate Nd2O2SO4. J. Coord. Chem. 2015, 68, 1865–1877. [Google Scholar] [CrossRef]
- Xue, J.S.; Antonio, M.R.; Soderholm, L. Polymorphs of Ln2MoO6: A neutron diffraction investigation of the crystal structures of La2MoO6 and Tb2MoO6. Chem. Mater. 1995, 7, 333–340. [Google Scholar] [CrossRef]
- Balestracci, R.; Mareschal, G. Etude structural des sulfates basiques de terres rares et d’yttrium. Mater. Res. Bull. 1967, 2, 993–998. [Google Scholar] [CrossRef]
- Haire, R.G.; Fahey, J.A. The oxysulfates and oxysulfides of americium, curium and berkelium. J. Inorg. Nucl. Chem. 1977, 39, 837–841. [Google Scholar] [CrossRef]
- Baybarz, R.D.; Fahey, J.A.; Haire, R.G. The preparation, crystal structures and some properties of califormium oxysulfate and oxysulfide. J. Inorg. Nucl. Chem. 1974, 36, 2023–2027. [Google Scholar] [CrossRef]
- Luu, S.D.N.; Vaqueiro, P. Synthesis, characterisation and thermoelectric properties of the oxytelluride Bi2O2Te. J. Solid State Chem. 2015, 226, 219–223. [Google Scholar] [CrossRef]
- Efremov, V.A.; Tyulin, A.V.; Trunov, V.K. Actual structure of tetragonal Ln2O2MoO4 and factors, determining the forming structure of the coordination polyhedra. Koord. Khim. 1987, 13, 1276–1282. [Google Scholar]
- Lü, M.; Colmont, M.; Kabbour, H.; Colis, S.; Mentré, O. Revised Bi/M layered oxo-sulfate (M = Co, Cu): A structural and magnetic study. Inorg. Chem. 2014, 53, 6969–6978. [Google Scholar] [CrossRef] [PubMed]
- Aurivillius, B. Crystal structures of ~[MI5Cl][Bi48O59Cl30], MI = Cu, Ag. Acta Chem. Scand. 1990, 44, 111–122. [Google Scholar] [CrossRef]
- Bruker-AXS APEX2. Version 2014.11–0; Bruker AXS Inc.: Madison, WI, USA, 2014.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, A71, 3–8. [Google Scholar]
- Petřiček, V.; Dušek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Krist.-Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Krivovichev, S.V.; Burns, P.C. The crystal chemistry of sulfate minerals. Rev. Miner. Geochem. 2000, 40, 1–112. [Google Scholar] [CrossRef]
- Tomaszewicz, E. Studies on reactivity in the solid state between some rare-earth metal oxides Ln2O3 where Ln = Y, La, Nd, Sm, Eu, Gd, Dy, Ho, Er, Lu and metal sulfates(VI) MSO4 where M = Ni, Cu, Zn, Cd. J. Mater. Sci. 2006, 41, 1675–1680. [Google Scholar] [CrossRef]




| Single-Crystal XRD | Powder XRD | |||
|---|---|---|---|---|
| [Bi2CuO3](SO4) | [Dy2O2](SO4) | [Ho2O2](SO4) | ||
| Space Group | C2/c | Space Group | C2/c | C2/c |
| a (Å) | 20.0283(7) | a (Å) | 13.3682(2) | 13.4172(1) |
| b (Å) | 5.3970(2) | b (Å) | 4.14721(5) | 4.15878(4) |
| c (Å) | 14.1413(5) | c (Å) | 8.0204(1) | 8.05626(8) |
| β, ° | 128.4450(10) | β, ° | 107.8070(8) | 107.6201(8) |
| V (Å3) | 1197.19(8) | V (Å3) | 423.35(1) | 428.44(1) |
| Dx | 6.941 | Dx | 7.18 | 7.024 |
| 2θ range (°). | 2.60–38.50 | 2θ range (°). | 10–120 | 10–120 |
| Rint | 0.030 | RP | 0.029 | 0.015 |
| R1 | 0.023 | RWP (%) | 0.043 | 0.014 |
| Gof | 1.040 | RF (%) | 0.029 | 0.035 |
| CCDC | 2021664 | CCDC | 2021867 | 2021874 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siidra, O.; Charkin, D.; Plokhikh, I.; Nazarchuk, E.; Holzheid, A.; Akimov, G. Expanding Family of Litharge-Derived Sulfate Minerals and Synthetic Compounds: Preparation and Crystal Structures of [Bi2CuO3]SO4 and [Ln2O2]SO4 (Ln = Dy and Ho). Minerals 2020, 10, 887. https://doi.org/10.3390/min10100887
Siidra O, Charkin D, Plokhikh I, Nazarchuk E, Holzheid A, Akimov G. Expanding Family of Litharge-Derived Sulfate Minerals and Synthetic Compounds: Preparation and Crystal Structures of [Bi2CuO3]SO4 and [Ln2O2]SO4 (Ln = Dy and Ho). Minerals. 2020; 10(10):887. https://doi.org/10.3390/min10100887
Chicago/Turabian StyleSiidra, Oleg, Dmitri Charkin, Igor Plokhikh, Evgeny Nazarchuk, Astrid Holzheid, and Georgy Akimov. 2020. "Expanding Family of Litharge-Derived Sulfate Minerals and Synthetic Compounds: Preparation and Crystal Structures of [Bi2CuO3]SO4 and [Ln2O2]SO4 (Ln = Dy and Ho)" Minerals 10, no. 10: 887. https://doi.org/10.3390/min10100887