Abstract
Purpose
Granulocyte colony stimulating factor (GCSF; also known as filgrastim) is a growth factor used to induce production of granulocytes. As the first locally developed and approved biosimilar medicine of Turkey, Fraven® being a biosimilar of filgrastim has been ab initio manufactured from cell to finished product at two different production facilities. Comprehensive structural, biological and functional characterization studies were performed to compare Fraven® from two different production sites and its reference product Neupogen® sourced from Turkey.
Methods
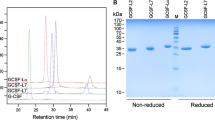
Primary and higher-order protein structures were analyzed by high performance liquid chromatography electrospray ionization-time of flight mass spectrometry, circular dichroism, and two-dimensional nuclear magnetic resonance spectroscopy. Isoelectric focusing, SDS-Page, size exclusion chromatography, and related proteins analyses were used to compare impurities. In order to assess functional similarity, surface plasmon resonance (SPR) was used. In vitro cell proliferation assay was also performed to show dose related drug response in NFS-60 cell line.
Results
Primary, secondary and tertiary structures of biosimilar Fraven® manufactured at both sites were found to be highly similar to the reference Neupogen®. Product related substances and impurities were also highly similar to the reference. Comparability of GCSF receptor binding affinities of each product was shown using the KD values of SPR analysis. In vitro cell proliferation similarity was also evaluated and proven by PLA.
Conclusion
Based on the similarity assessment, despite being manufactured at two different production sites, biosimilar Fraven® is highly similar to the reference product Turkey originated Neupogen®.








Similar content being viewed by others
References
Fukunaga R, Ishizaka-Ikeda E, Nagata S. Purification and characterization of the receptor for murine granulocyte colony-stimulating factor. J Biol Chem. 1990;265(23):14008–15.
Bendall LJ, Bradstock KF. G-CSF: from granulopoietic stimulant to bone marrow stem cell mobilizing agent. Cytokine Growth Factor Rev. 2014. https://doi.org/10.1016/j.cytogfr.2014.07.011.
Herman AC, Boone TC, Lu HS. Characterization, formulation, and stability of Neupogen® (Filgrastim), a recombinant human GranulocyteColony stimulating factor. In: Pearlman R, Wang YJ, editors. Formulation, characterization, and stability of protein drugs. New York: Plenum Press; 1996. p. 303–28.
Mantovani M, Caruso C, Facchini F, Pascon R, Cagnacci P, Magalhaes V. Physicochemical and biological comparison of the first Brazilian biosimilar filgrastim with its reference product. Biosimilars. 2016. https://doi.org/10.2147/BS.S107898.
Sörgel F, Lerch H, Lauber T. Physicochemical and biologic comparability of a biosimilar granulocyte Colony-stimulating factor with its reference product. BioDrugs. 2010. https://doi.org/10.2165/11585100-000000000-00000.
Cornes P, Krendyukov A. The evolution of value with filgrastim in oncology. Future Oncol. 2019. https://doi.org/10.2217/fon-2018-0762.
Awad M, Singh P, Hilas O. Zarxio (Filgrastim-sndz): the first biosimilar approved by the FDA. PT. 2017;42(1):19–23.
Schiestl M, Zabransky M, Sörgel F. Ten years of biosimilars in Europe: development and evolution of the regulatory pathways. Drug Des Devel Ther. 2017. https://doi.org/10.2147/DDDT.S130318.
Filgrastim concentrated solution [monograph no. 07/2019:2206]. In: European Pharmacopoeia. 10th ed. Suppl. 6.8. Strasbourg: European Directorate for the Quality of Medicines & HealthCare (EDQM).
Filgrastim injection [monograph no. 07/2019:2848]. In: European Pharmacopoeia. 10th ed. Suppl. 10.0. Strasbourg: European Directorate for the Quality of Medicines & HealthCare (EDQM).
Greenfield NJ. Using circular dichroism spectra to estimate protein secondary structure. Nat Protoc. 2006. https://doi.org/10.1038/nprot.2006.202.
Mire-Sluis AR, Gerrard T, Das RG, Padilla A, Thorpe R. Biological assays: their role in the development and quality control of recombinant biological medicinal products. Biologicals. 1996. https://doi.org/10.1006/biol.1996.0050.
Hulme EC, Trevethick MA. Ligand binding assays at equilibrium: validation and interpretation. Br J Pharmacol. 2010. https://doi.org/10.1111/j.1476-5381.2009.00604.x.
Pollard TD. A guide to simple and informative binding assays. Mol Biol Cell. 2010. https://doi.org/10.1091/mbc.E10-08-0683.
ICH Q5e Comparability of biotechnological/biological products. European medicines agency. In: CPMP/ICH/5721/03; 2005.
Acknowledgments
All analyses were funded by Arven and partially by Scientific and Technological Research Council of Turkey (TUBITAK; 3100431). Study design, management, analysis, and interpretation of analytical data were done by the authors and other Arven personnel. We gratefully acknowledge our former team members who also contributed to the development of the biosimilar filgrastim, Fraven®.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bor Tekdemir, Z., Seckin, A.I., Kacar, T. et al. Evaluation of Structural, Biological, and Functional Similarity of Biosimilar Granulocyte Colony Stimulating Factor to its Reference Product. Pharm Res 37, 215 (2020). https://doi.org/10.1007/s11095-020-02932-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02932-7