Abstract
Purpose
Polysorbate 20 (PS20), a commonly used surfactant in biopharmaceutical formulations, can undergo hydrolytic degradation resulting in free fatty acids (FFAs) that precipitate to form particles. This work investigates the ability for silicone oil (si-oil) coated on the interior walls of prefilled syringes (PFSs) to act as a sink for FFAs and potentially delay FFA particle formation.
Methods
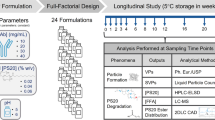
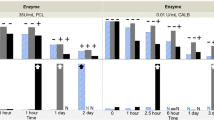
Myristic acid distribution coefficient was measured in a two-phase system containing si-oil and formulation buffer at a range of aqueous conditions. An empirical model was built from these data to predict distribution coefficient based on aqueous conditions. To verify the model, PS20 was degraded using model lipases side-by-side in glass vials and PFSs while monitoring sub-visible particles.
Results
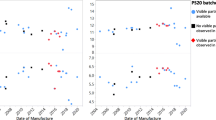
The empirical model demonstrates that the partitioning of myristic acid into si-oil is maximized at low pH and low PS20 concentration. The model predicts that the presence of si-oil at levels typical in PFSs provides at most an 8.5% increase in the total carrying capacity for myristic acid compared to a non-coated glass vial. The time to onset of FFA particles was equivalent between degradations performed in two PFS models coated with differing levels of silicone oil and in non-coated glass vials.
Conclusion
Herein, we demonstrate that FFAs partition from aqueous solution into si-oil. However, the extent of the partitioning effect is not large enough to delay PS20-related FFA particle formation at typical formulation conditions (pH 5.0–7.5, 0.01% - 0.1% w/v PS20) filled in typical PFSs (<1.0 mg si-oil/mL aqueous fill).









Similar content being viewed by others
Abbreviations
- FFA:
-
Free fatty acid
- PFS:
-
Pre-filled syringe
- PS20:
-
Polysorbate-20
- SI-OIL:
-
Silicone oil
- FB:
-
Formulation buffer
- D:
-
Distribution coefficient
- P:
-
Partition coefficient
- PCL:
-
Pseudomonas cepacea lipase
- CALB:
-
Candida antarctica lipase B
- RP-UHPLC:
-
Reverse-phase ultra-high performance liquid chromatography
- MM-HPLC:
-
Mixed-mode high performance liquid chromatography
- ELSD:
-
Evaporative light scattering detection
- RP-HPLC:
-
Reverse-phase high performance liquid chromatography
- HA:
-
Protonated (non-ionized) fatty acid
- A− :
-
Deprotonated (ionized) fatty acid
References
Manning MC, Patel K, Borchardt RT. Stability of protein pharmaceuticals. Pharm Res. 1989;6(11):903–18.
Duncan MR, Lee JM, Warchol MP. Influence of surfactants upon protein/peptide adsorption to glass and polypropylene. Int J Pharm. 1995;120(2):179–88.
Wang W. Instability, stabilization, and formulation of liquid protein pharmaceuticals. Int J Pharm. 1999;185(2):129–88.
Jorgensen L, Hostrup S, Moeller EH, Grohganz H. Recent trends in stabilising peptides and proteins in pharmaceutical formulation – considerations in the choice of excipients. Expert Opinion on Drug Delivery. 2009;6(11):1219–30.
Kerwin BA. Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: structure and degradation pathways. J Pharm Sci. 2008;97(8):2924–35.
Ha E, Wang W, Wang YJ. Peroxide formation in polysorbate 80 and protein stability. J Pharm Sci. 2002;91(10):2252–64.
Kishore RSK, Kiese S, Fischer S, Pappenberger A, Grauschopf U, Mahler H-C. The degradation of Polysorbates 20 and 80 and its potential impact on the stability of biotherapeutics. Pharm Res. 2011;28(5):1194–210.
Labrenz SR. Ester hydrolysis of Polysorbate 80 in mAb drug product: evidence in support of the hypothesized risk after the observation of visible particulate in mAb formulations. J Pharm Sci. 2014;103(8):2268–77.
Tomlinson A, Demeule B, Lin B, Yadav S. Polysorbate 20 degradation in biopharmaceutical formulations: quantification of free fatty acids, characterization of particulates, and insights into the degradation mechanism. Mol Pharm. 2015;12(11):3805–15.
Dixit N, Salamat-Miller N, Salinas PA, Taylor KD, Basu SK. Residual host cell protein promotes Polysorbate 20 degradation in a Sulfatase drug product leading to free fatty acid particles. J Pharm Sci. 2016;105(5):1657–66.
Kishore RSK, Pappenberger A, Dauphin IB, Ross A, Buergi B, Staempfli A, et al. Degradation of polysorbates 20 and 80: studies on thermal autoxidation and hydrolysis. J Pharm Sci. 2011;100(2):721–31.
Dwivedi M, Blech M, Presser I, Garidel P. Polysorbate degradation in biotherapeutic formulations: identification and discussion of current root causes. Int J Pharm. 2018;552(1):422–36.
Doshi N, Demeule B, Yadav S. Understanding particle formation: solubility of free fatty acids as Polysorbate 20 degradation byproducts in therapeutic monoclonal antibody formulations. Mol Pharm. 2015;12(11):3792–804.
Saggu M, Liu J, Patel A. Identification of subvisible particles in biopharmaceutical formulations using Raman spectroscopy provides insight into Polysorbate 20 degradation pathway. Pharm Res. 2015;32(9):2877–88.
Chiu J, Valente KN, Levy NE, Min L, Lenhoff AM, Lee KH. Knockout of a difficult-to-remove CHO host cell protein, lipoprotein lipase, for improved polysorbate stability in monoclonal antibody formulations. Biotechnol Bioeng. 2017;114(5):1006–15.
Katz JS, Nolin A, Yezer BA, Jordan S. Dynamic properties of novel excipient suggest mechanism for improved performance in liquid stabilization of protein biologics. Mol Pharm. 2019;16(1):282–91.
Makwana S, Basu B, Makasana Y, Dharamsi A. Prefilled syringes: an innovation in parenteral packaging. Int J Pharm Investig. 2011;1(4):200–6.
Sacha G, Rogers JA, Miller RL. Pre-filled syringes: a review of the history, manufacturing and challenges. Pharm Dev Technol. 2015;20(1):1–11.
Sigma-Aldrich. Silicone Oil Safety Data Sheet (Aldrich - 378364). 2020.
Doshi N, Fish R, Padilla K, Yadav S. Evaluation of super refined™ Polysorbate 20 with respect to Polysorbate degradation. Journal of Pharmaceutical Sciences: Particle Formation and Protein Stability; 2020.
Hewitt D, Zhang T, Kao YH. Quantitation of polysorbate 20 in protein solutions using mixed-mode chromatography and evaporative light scattering detection. J Chromatogr A. 2008;1215(1–2):156–60.
Hewitt D, Alvarez M, Robinson K, Ji J, Wang YJ, Kao YH, et al. Mixed-mode and reversed-phase liquid chromatography-tandem mass spectrometry methodologies to study composition and base hydrolysis of polysorbate 20 and 80. J Chromatogr A. 2011;1218(15):2138–45.
McShan AC, Kei P, Ji JA, Kim DC, Wang YJ. Hydrolysis of Polysorbate 20 and 80 by a range of Carboxylester hydrolases. PDA J Pharm Sci Technol. 2016;70(4):332–45.
Siska CC, Pierini CJ, Lau HR, Latypov RF, Fesinmeyer RM, Litowski JR. Free fatty acid particles in protein formulations, part 2: contribution of polysorbate raw material. J Pharm Sci. 2015;104(2):447–56.
Werk T, Volkin DB, Mahler HC. Effect of solution properties on the counting and sizing of subvisible particle standards as measured by light obscuration and digital imaging methods. Eur J Pharm Sci. 2014;53:95–108.
Barroso da Silva FL, Bogren D, Söderman O, Åkesson T, Jönsson B. Titration of fatty acids solubilized in cationic, nonionic, and anionic micelles. Theory and experiment. The Journal of Physical Chemistry B. 2002;106(13):3515–22.
Popović MR, Popović GV, Agbaba DD. The effects of anionic, cationic, and nonionic surfactants on Acid–Base Equilibria of ACE inhibitors. J Chem Eng Data. 2013;58(9):2567–73.
Tomlinson A, Zarraga IE, Demeule B. Characterization of Polysorbate Ester fractions and implications in protein drug product stability. Mol Pharm. 2020;17(7):2345–53.
Bravo B, Sánchez J, Cáceres A, Chávez G, Ysambertt F, Márquez N, et al. Partitioning of fatty acids in oil/water systems analyzed by HPLC. J Surfactant Deterg. 2008;11(1):13–9.
Sangster J. Octanol-water partition coefficients of simple organic compounds. J Phys Chem Ref Data. 1989;18(3):1111–229.
LOGKOW databank [Internet]. Sangster Res. Lab. 1994.
Funke S, Matilainen J, Nalenz H, Bechtold-Peters K, Mahler HC, Friess W. Optimization of the bake-on siliconization of cartridges. Part I: optimization of the spray-on parameters. Eur J Pharm Biopharm. 2016;104:200–15.
Jones LS, Kaufmann A, Middaugh CR. Silicone oil induced aggregation of proteins. J Pharm Sci. 2005;94(4):918–27.
Lapelosa M, Patapoff TW, Zarraga IE. Molecular simulations of micellar aggregation of polysorbate 20 ester fractions and their interaction with N-phenyl-1-naphthylamine dye. Biophys Chem. 2016;213:17–24.
Nayem J, Zhang Z, Tomlinson A, Zarraga IE, Wagner NJ, Liu Y. Micellar morphology of Polysorbate 20 and 80 and their Ester fractions in solution via small-angle neutron scattering. J Pharm Sci. 2020;109(4):1498–508.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fish, R., Lin, J. & Doshi, N. Impact of Silicone Oil on Free Fatty Acid Particle Formation due to Polysorbate 20 Degradation. Pharm Res 37, 216 (2020). https://doi.org/10.1007/s11095-020-02936-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02936-3