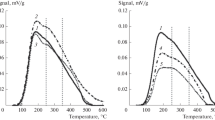
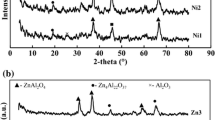
Abstract
Platinum catalysts supported onto bayerite alumina were synthesized and studied. Alumina of the θ-Al2O3 type, which was prepared from metallic aluminum by an aluminate method, was used as a support, and platinum(IV) nitrate and hydrogen hexachloroplatinate(IV) precursors containing Pt4+ ions were used for supporting an active component. The catalysts were studied by physicochemical methods such as X-ray phase analysis, X-ray photoelectron spectroscopy, transmission electron microscopy, and ultraviolet-visible diffuse reflection spectroscopy. It was found that platinum occurred in a finely dispersed state in both of the catalysts, and it was uniformly distributed over the support surface. The particle sizes were 2.8 and 0.9 nm in the samples synthesized from platinum(IV) nitrate and hydrogen hexachloroplatinate(IV), respectively. The most uniform surface distribution, high dispersity, and a narrow particle-size distribution were obtained with the use of hydrogen hexachloroplatinate(IV). The catalysts were tested in the partial oxidation of natural gas. The catalyst synthesized using platinum(IV) nitrate afforded an almost thermodynamically equilibrium distribution of the reaction products of partial oxidation at a temperature of 700–800°С.






Similar content being viewed by others
REFERENCES
York, A.P.E., Xiao, T., and Green, M.L.H., Top. Catal., 2003, vol. 22, nos. 3–4, p. 345.
Faravelli, T., Goldaniga, A., Ranzi, E., Dietz, A., Davis, M., and Schmidt, L.D., Stud. Surf. Sci. Catal., 1998, vol. 119, p. 575.
Bukhtiyarov, V.I., Moroz, B.L., Bekk, I.E., and Prosvirin, I.P., Katal. Prom-sti, 2008, Special Issue, p. 44.
O’Connell, M., Kolb, G., Zapf, R., Men, Y., and Hessel, V., Catal. Today, 2009, vol. 144, p. 306.
Belyi, A.S., Kinet. Catal., 2008, vol. 49, no. 4, p. 562.
Moroz, E.M., Russ. Chem. Rev., 1992, vol. 61, no. 2, p. 188.
Ushakov, V.A., Moroz, E.M., Zhdan, P.A., Boronin, A.I., Bursian, N.R., Kogan, S.B., and Levitskii, E.A., Kinet. Katal., 1978, vol. 19, no. 3, p. 744.
Hickman, D.A. and Schmidt, L.D., J. Catal., 1992, vol. 138, p. 267.
Carlsson, P.-A., Fridell, E., and Skoglundha, M., Catal. Lett., 2007, vol. 115, nos. 1–2, p. 1.
O’Brien, C.P., Jenness, G.R., Dong, H., Vlachos, D.G., and Lee, I.C., J. Catal., 2016, vol. 337, p. 122.
Yazawa, Y., Takagi, N., Yoshida, H., Komai, S.-I., Satsuma, A., Tanaka, T., Yoshida, S., and Hattori, T., Appl. Catal., A, 2002, vol. 233, p. 103.
Yazawa, Y., Yoshida, H., and Hattori, T., Appl. Catal., A, 2002, vol. 237, p. 139.
Liu, Z.G., Berg, D.R., Vasys, V.N., Dettmann, M.E., Zielinska, B., and Schauer, J.J., Atmos. Environ., 2010, vol. 44, p. 1108–1115.
Stiles, A.B., Catalyst Supports and Supported Catalysts: Theoretical and Applied Concepts, Oxford: Butterworth-Heinemann, 1987.
Lippens, B.C. and Steggerda, J.J., Active Alumina, Linsen, B., Ed., London: Academic, 1970.
Tsybulya, S.V. and Kryukova, G.N., Phys. Rev. B, 2008, vol. 77, p. 024112-1–024112-13.
Busca, G., Catal. Today, 2014, vol. 226, p. 2.
Carrera, A., Pelucchi, M., Stagni, A., Beretta, A., and Groppi, G., Int. J. Hydrogen Energy, 2017, vol. 42, no. 39, p. 24675.
Bodke, A.S., Bharadwaj, S.S., and Schmidt, L.D., J. Catal., 1998, vol. 179, p. 138.
Park, J.E., Kim, B.B., and Park, E.D., Korean J. Chem. Eng., 2015, vol. 32, no. 11, p. 2212.
Knozinger, H. and Ratnasamy, P., Rev. Sci. Eng., 1978, vol. 17, p. 31.
Tregubenko, V.Yu., Cand. Sci. (Chem.) Dissertation, Omsk: Institute of Problems of Refining of Hydrocarbons SB RAS, 2011.
Della Gatta, G., Fubini, B., Ghiotti, G., and Morterra, C., J. Catal., 1976, vol. 43, p. 90.
Bolis, V., Cerrato, G., Magnacca, G., Morterra, C., Bolis, V., Cerrato, G., Magnacca, G., and Morterra, C., Thermochim. Acta, 1998, vol. 312, p. 63.
Saalfeld, H., Neues Jarb. Mineral. Ahandl., 1960, vol. 95, p. 1.
Zhou, R.-S. and Snyder, R.L., Acta Crystallogr., 1991, vol. 47, p. 617.
Patterson, A.L., Phys. Rev. 1939, vol. 56, p. 978.
Andersen, A.G., Hayakawa, T., Tsunoda, T., Orita, H., Shimizu, M., and Takehira, K., Catal. Lett., 1993, vol. 18, nos. 1–2, p. 37.
Rogozhnikov, V.N., Snytnikov, P.V., Salanov, A.N., Kulikov, A.V., Ruban, N.V., Potemkin, D.I., Sobyanin, V.A., and Kharton, V.V., Mater. Lett., 2019, vol. 236, p. 316.
Frade, J.R., Kharton, V.V., Yaremchenko, A.A., Tsipis, E.V., Shaula, A.L., Naumovich, E.N., Kovalevsky, A.V., and Marques, F.M.B., Bol. Soc. Esp. Ceram., 2004, vol. 43, issue 3, p. 640.
Bokii, G.B., Kristallokhimiya (Crystallochemistry), Moscow: Nauka, 1971.
Ershov, B.G., Russ. Chem. Bull., 2001, vol. 50, no. 4, p. 626.
Gololobov, A.M., Bekk, I.E., Bragina, G.O., Zaikovskii, V.I., Ayupov, A.B., Telegina, N.S., Bukhtiyarov, V.I., and Stakheev, A.Yu., Kinet. Katal., 2009, vol. 50, no. 6, p. 830.
Funding
This work was supported by the Russian Foundation for Basic Research, project no. 19-03-00595.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by V. Makhlyarchuk
Abbreviations: XPS, X-ray photoelectron spectroscopy; TEM, transmission electron microscopy; UV-Vis DRS, ultraviolet-visible diffuse reflectance spectroscopy; CSR, coherent scattering region.
Rights and permissions
About this article
Cite this article
Shefer, K.I., Kovtunova, L.M., Rogozhnikov, V.N. et al. Influence of the Preparation Method on the Physicochemical and Catalytic Properties of Platinum Catalysts Supported on Bayerite Alumina for the Partial Oxidation Reactions of Hydrocarbons. Kinet Catal 61, 801–808 (2020). https://doi.org/10.1134/S0023158420050092
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158420050092