Morphology, Activation, and Metal Substitution Effects of AlPO4-5 for CO2 Pressure Swing Adsorption
- 1Department of Chemical Engineering, Khalifa University, Abu Dhabi, United Arab Emirates
- 2School of Chemical Engineering, National Technical University of Athens, Athens, Greece
- 3Center for Catalysis and Separations (CeCaS), Khalifa University, Abu Dhabi, United Arab Emirates
- 4Research and Innovation Center on CO2 and H2 (RICH), Khalifa University, Abu Dhabi, United Arab Emirates
Aluminophosphate, AlPO4-5, an AFI zeotype framework consisting of one-dimensional parallel micropores, and metal-substituted AlPO4-5 were prepared and studied for CO2 adsorption. Preparation of AlPO4-5 by using different activation methods (calcination and pyrolysis), incorporation of different metals/ions (Fe, Mg, Co, and Si) into the framework using various concentrations, and manipulation of the reaction mixture dilution rate and resulting crystal morphology were examined in relation to the CO2 adsorption performance. Among the various metal-substituted analogs, FeAPO-5 was found to exhibit the highest CO2 capacity at all pressures tested (up to 4 bar). Among the Fe-substituted samples, xFeAPO-5, with x being the Fe/Al2O3 molar ratio in the synthesis mixture (range of 2.5:100–10:100), 5FeAPO-5 exhibited the highest capacity (1.8 mmol/g at 4 bar, 25°C) with an isosteric heat of adsorption of 23 kJ/mol for 0.08–0.36 mmol/g of CO2 loading. This sample also contained the minimum portion of extra-framework or clustered iron and the highest mesoporosity. Low water content in the synthesis gel led to the formation of spherical agglomerates of small 2D-like crystallites that exhibited higher adsorption capacity compared to columnar-like crystals produced by employing more dilute mixtures. CO2 adsorption kinetics was found to follow a pseudo–first-order model. The robust nature of AlPO4-5–based adsorbents, their unique one-dimensional pore configuration, fast kinetics, and low heat of adsorption make them promising for pressure swing adsorption of CO2 at industrial scale.
Introduction
The severe environmental concern related to the greenhouse effects is mainly attributed to gases that absorb and emit radiation within the thermal infrared range. The primary greenhouse gases are carbon dioxide, methane, water vapor, nitrous oxide, and ozone. CO2 is the most important contributor coming mainly from combustion of fossil fuels and being released into the atmosphere from the power plants, steel plants, cement industry, and other large-scale industrial operations, as well as from transportation (Kontos et al., 2014). Notably, the increase of total primary energy supply, reaching 150% in 2014 compared to 1971, is caused by the associated worldwide economic growth with fossil fuels holding the most significant share for that energy produced (Metz et al., 2005; IEA, 2016; Tabish et al., 2020). Under these circumstances, it is evident that carbon dioxide emissions will keep increasing as energy demands will continue being covered primarily by fossil fuels at least for the next 50 years.
CO2 capture, utilization, and storage (CCUS) technologies have been proposed to stabilize the concentrations of greenhouses gases in the atmosphere and mitigate their impact. Among other proposed solutions, such as retrofits of existing units, usage of fuels with less carbon dioxide footprint, and nuclear and renewable energy, CCUS is the most promising one in terms of compatibility with the energy production, continued dependence on fossil fuels, and delivery infrastructure. Focusing on postcombustion, different technologies have been used. Employing amine-based solvents is the most mature technology. Monoethanolamine, diethanolamine, and N-methyldiethanolamine are commonly used alkanolamines. These lean amines, commonly diluted along with water of content in the order of about 70%, have a high reactivity toward CO2. Still, the technology has certain drawbacks mainly associated with high energy consumption for the regeneration step, requirement of large voluminous equipment, high corrosion rates, and solvents slippage to the atmosphere (Resnik et al., 2004; Haszeldine, 2009). As promising alternatives to solvent-based systems, which have constituted the main industrial practice for several decades, technologies based on adsorption and membrane separation are gaining considerable attention (Pilatos et al., 2010; Labropoulos et al., 2015; Kueh et al., 2018). Concerning adsorption, emphasis is put on porous materials. These can be divided into two categories: (i) physical adsorbents, such as porous carbons, zeolites, and metal–organic frameworks, and (ii) chemical adsorbents, such as functionalized materials with surface agents such as amine moieties (Choi et al., 2009; Sayari et al., 2011; Pokhrel et al., 2018; Varghese and Karanikolos, 2020). In this front, various materials are being discovered and explored, yet a platform of suitable materials/systems to treat a wide range of industrial emissions at a large scale still remains a challenge. The reason is that multiple factors need to be met at the same time, i.e., adsorbents need to exhibit (i) high capacity; selectivity compared to other components such as NOx, SO2, and H2O vapor; fast adsorption/desorption kinetics; and low energy consumption; and (ii) chemical and thermal stability, sustainable performance for many cycles, low manufacturing cost, and mechanical robustness at large scale.
Zeolites have been studied for CO2 capture, particularly involving dry CO2, based on their relatively high adsorption capacity (Chue et al., 1995; Sircar and Golden, 1995; Siriwardane et al., 2001; Chou and Chen, 2004), low-cost of production, and excellent thermal stability (Musyoka et al., 2015). Yet, due to polar zeolite surfaces, these materials are highly hydrophilic resulting in lower CO2 capture capacity and selectivity and early saturation in the presence of moisture (Corma, 2003). To battle this problem, less hydrophilic zeolite-type materials need to be explored. Aluminophosphates (AlPOs) are among the most notable adsorbents for this duty. AlPOs were discovered in 1982 by Wilson and partners of Union Carbide (Wilson et al., 1982). The framework of AlPOs, such as AlPO4-5, consists of alternating Al3+ and P5+ connected by oxygen atoms (Wilson et al., 1982; Stoeger et al., 2012). The main feature is the charge neutrality of the framework, which occurs from the equal ratio of alumina to phosphorus (Al/P = 1). This constant ratio producing materials of a net neutral electric charge prevents ion exchange, and as a result, AlPOs tend to be only slightly hydrophilic due to the absence of acidic sites (Cundy and Cox, 2003; Carmine, 2012). Indeed, in our recent work, we showed that AlPO4-5 is rather hydrophobic particularly at relatively low water partial pressures, where water molecules occupy niches close to pore walls, followed later by the filling of the central pore area (Schlegel et al., 2018). However, AlPOs retain their CO2 sorption capacity potential due to the high concentration of physisorption sites. Therefore, the limited hydrophilicity, concentration of physisorption sites, and more linear shape of the CO2 adsorption isotherms as compared to other zeolites suggest that these materials would have a longer process lifetime extending over many adsorption/regeneration cycles by pressure-mediated tuning between adsorption/desorption cycles [pressure swing adsorption (PSA)]. Liu et al. (2011) examined 8-member ring AlPOs (AlPO4-17, AlPO4-18, AlPO4-53, and AlPO4-25) at different temperatures and determined CO2 uptake capacities in the range of 1.52–2.32 mmol/g at 273–293 K and 100 kPa. Among the above structures, AlPO4-53 possessed a higher CO2 affinity and a CO2/N2 selectivity of 98.4 with low interaction with water molecules as compared to the benchmark zeolite 13X. Zhao et al. (2009) investigated AlPO4-14 and reported CO2 adsorption capacities ranging from 2.0 to 2.7 mmol/g within the temperature range of 273–300 K at 100 kPa, exhibiting also relatively high CO2 over CH4 selectivity. Delgado et al. (2013) studied the adsorption behavior of AlPO4-11 for CO2, CH4 and N2 and reported a CO2 adsorption capacity of 0.31-0.7 mmol/g at 298–338 K and 100 kPa.
In addition to high adsorption capacity and selectivity, fast adsorption/desorption kinetics and a low heat of adsorption are key factors for an industrially prominent adsorbent candidate in PSA CO2 capture applications. The work reported herein studies the synthesis and modification/functionalization of AlPO4-5 and its metal substituted analogs as potential candidates for CO2 capture. The crystal lattice of AlPO4-5 possesses a hexagonal symmetry and a monodirectional channel morphology extending along the c-axis. The main channels are created by 12-member rings of alternating tetrahedra of [AlO4]− and [PO4]+ having a diameter of 7.2 Å (Rajic and Kaucic, 2002; Guo et al., 2005; Karanikolos et al., 2008). Here, we assess the impact of various factors on CO2 sorption, namely, (a) the effect of various heteroatoms used in isomorphic substitution in the AlPO4-5 framework at varying concentrations, (b) the pore activation method and in particular the affinity of remnant carbon species present into the inner pore surface after structure-directing agent (SDA) removal by two different thermal treatment methods, i.e., partial oxidation and pyrolysis, and (c) the impact of AlPO4-5 crystal morphology and hydrothermal synthesis mixture composition and in particular the water content in the mixture.
Experimental Section
Materials
Aluminum isopropoxide (Merck), orthophosphoric acid (85% in H2O, Sigma Aldrich), and triethylamine (TEA, Merck) were used as precursors for AlPO4-5 growth. Tetraethyl orthosilicate (Merck), magnesium chloride (Merck), cobalt (II) acetate tetrahydrate (98%, Sigma-Aldrich), and iron (III) nitrate non-ahydrate (Merck) were used as metal precursors for the synthesis of the metal-substituted AlPOs (MeAPO-5).
Growth of AlPO4-5 and MeAPO-5 Adsorbents
The AlPO materials were grown hydrothermally from reaction mixtures starting from a gel composition of 1Al2O3:1.3P2O5:1.2TEA:xMe:yH2O, where x and y refer to the metal/Al2O3 and water/Al2O3 molar ratios, respectively. Desired amount of aluminum isopropoxide was dissolved in deionized water under stirring for 3 h. To this solution, orthophosphoric acid was added dropwise, and the mixture was stirred for another 1 h. TEA, as the SDA, was then added dropwise, and the mixture was stirred for 24 h. For the preparation of the metal or ion substituted AlPOs, the metal precursor was added right after the addition of the SDA. The reaction gel having a pH ranging from 5 to 6 was transferred to a Teflon-lined stainless steel autoclave and placed inside a preheated oven at a temperature of 160°C for 24 h. After growth, the autoclave was quenched, and the solid product was collected after repeated centrifugation/washing cycles. The obtained crystals were dried at 80°C for 6 h and were subsequently calcined in air using a ramping rate of 2.5°C/min until temperature reached 80°C, keeping temperature stable for 30 min there, and further increasing it up to 600°C, where it was kept constant for 5.5 h. Activation at various temperatures under air flow (calcination) or nitrogen (pyrolysis) in a tubular furnace was also performed in order to parametrically explore decomposition/removal of the SDA occluded into the pores using the above temperature ramping program. A water/Al2O3 molar ratio of 100:1 was used in these experiments. The MeAPOs were synthesized using different metals/ions (Fe, Mg, Co, and Si) into the AlPO4-5 framework with water/Al2O3 molar ratio of 100:1 and metal/Al2O3 ratio of 5:100. For the FeAPO-5 adsorbents, additional metal contents were studied as well.
The following naming code of samples was applied throughout the various sets of experiments in this work:
For the activation set of experiments: TAlPO4-5.P and TAlPO4-5.C, where T is the thermal treatment temperature in °C, and P and C stand for pyrolysis under inert atmosphere and calcination under airflow, respectively.
For the metal substitution set of experiments: The molar ratios were placed ahead of the sample names. For example, 5FeAPO-5 indicates a metal/Al2O3 ratio of 5:100.
For the experiments on varying water content in the synthesis mixture: The H2O/Al2O3 molar ratio was placed ahead of the sample names. For example, 400AlPO4-5 indicates a H2O/Al2O3 ratio of 400:1.
Characterization
The crystallinity of the synthesized samples was investigated by X-ray diffraction (XRD) using a Panalytical X'Pert Pro Powder Diffractometer. A Cu-Kα monochromatized radiation source with wavelength λ = 1.5406 Å, power 40 kV, and current of 40 mA was utilized, and the scan speed was set to 0.02 degrees/s. Morphology evaluation was performed by scanning electron microscopy (SEM) using an FEI Quanta 200 microscope. Samples were placed on a carbon tape and were coated by gold to enhance the conductivity and to allow observation at 30 kV. Ultraviolet-visible diffuse reflectance spectroscopy (UV-Vis DRS) of the adsorbents was recorded using a Varian Analytical Cary 5000 UV-Vis-NIR spectrometer, equipped with diffuse reflectance accessory used to record electronic spectra from 200 to 800 nm. Thermogravimetric analysis (TGA) was performed using TA instruments Trios V3.1 analyzer under 50 mL/min of air flow with a ramping rate of 10°C/min from 25 to 900°C. Fourier-transform infrared spectroscopy (FTIR) was carried out on a BRUKER TENSOR II series FTIR spectrometer with the aid of diamond attenuated total reflectance crystal, where thin sections of the samples were scanned in the wavenumber region of 4,000 to 400 cm−1 with a resolution of 4 cm−1 by undergoing 32 scans. Nitrogen adsorption–desorption analysis was performed at 77 K using a Micromeritics 3-Flex analyzer. Before the analysis, the samples were degassed at 220°C for 3 h. The surface area was determined by the Brunauer–Emmett–Teller (BET) method. Pore size distribution and pore volume were obtained by processing adsorption–desorption data using the Barrett, Joyner, Halenda (BJH) and Horvath–Kawazoe (HK) models via the MicroActive software.
CO2 Adsorption
CO2 adsorption experiments were carried out using a Rubotherm gravimetric sorption analyzer (IsoSORP STATIC 3xV-MP). The magnetic suspension balance measures sample weight and gas dosing to determine the adsorption equilibrium. Approximately 50 mg of each activated adsorbent was first heated under vacuum at 150°C for 3 h to remove moisture and other volatile substances until a constant sample mass was obtained. Buoyancy correction was performed using helium gas and incorporated into the sample weight. The sample in a stainless-steel holder was pressurized with CO2 gas up to 4 bar (in steps of 1 bar), and continuous measurement of sample mass gain during CO2 adsorption was determined at 25°C under equilibrium/saturation conditions. The gravimetric analyzer software uses the recorded mass changes to estimate the sorption capacity at each pressure step and incorporates the buoyancy correction as well in order to generate the final CO2 sorption isotherm. For the adsorption kinetics study, the adsorbents were measured again but with a single step directly up to 4 bar taking capacity values at regular time intervals. The procedure was repeated for three different temperatures, namely, 25, 45, and 60°C.
Results and Discussion
Activation of Adsorbents by Calcination and Pyrolysis
Upon growth of porous crystalline materials such as zeolites, SDAs (or organic templates) are typically used to direct the formation of the pores. Following growth, removal of these molecules from the pores needs to take place as to activate the open porosity of the materials. Whether the occluded molecules are completely decomposed and removed or remnants of carbon still exist into the pores may affect the affinity and interaction of the internal surface with CO2. This was first investigated in our study by activating the resulting AlPO4-5 crystals via calcination and partial calcination (air flow) or pyrolysis (nitrogen atmosphere) at various temperatures. The latter was employed based on our previous work revealing that graphitic carbon in the form of carbon nanotubes (CNTs) into the pores of oriented AlPO4-5 films affected CO2 sorption, as well as permeance behavior through the resulting CNT membranes (Labropoulos et al., 2015). In addition, the occluded amine SDA is partially decomposed and removed upon partial calcination, which might create extra spacing/porosity within the larger AlPO4-5 channels. The objective was to examine whether remaining carbon species would act favorably toward CO2 adsorption via interaction of CO2 with both AlPO4-5 and carbon surfaces, or complete removal of SDA would be preferred due to maximizing the pore volume and carbon-free AlPO4-5 surface.
Consequently, for calcination, five different temperatures were used, namely, 300, 400, 500, 600, and 700°C under airflow. The distinct color of the materials produced at the different calcination temperatures is shown in Supplementary Figure 1. The as-synthesized AlPO4-5 crystals had a white color. The material calcined between 300 and 500°C had a distinct brown coloration that turned softer as the temperature increased, which indicates the existence of remaining carbon species at the external crystal surface at these relatively low calcination temperatures with carbon content being decreased with increase in temperature. At temperatures above 600°C the materials turned white again, indicating complete carbon removal.
Pyrolysis of the synthesized AlPO4-5 samples was carried out under nitrogen flow at temperatures of 240, 400, and 700°C. The aim was to investigate possible affinity enhancement effects between CO2 and carbon species resulting from the TEA pyrolysis in the pores or formation of additional carbon porosity/surface within the AFI channels. The creation of porous carbon-based structure and possibly extra porosity inside the channels of AlPO4-5 might enhance affinity toward CO2 due to the existence of pyrolytic carbon. Analogous treatment yielded formation of single-wall CNTs inside the AFI pores of powder AlPO4-5 crystals (Tang et al., 1998), as well as in oriented membrane configuration for membrane-based gas separation, as demonstrated in our previous work (Labropoulos et al., 2015). The pyrolysis temperature of 240°C was selected as it represents a point within the TEA decomposition region based on TGA data (Wan et al., 2000).
XRD analysis of selected calcined and pyrolyzed samples are shown in Figure 1A. The water/Al2O3 molar ratio was fixed at 100 for this activation effect study. All samples treated at temperatures up to 700°C exhibit the characteristic diffraction peaks of AFI (Karanikolos et al., 2008; Basina et al., 2018), confirming the stability and structural integrity of the materials up to this temperature. Notably, the sample pyrolyzed at the lowest temperature (240°C), in addition to the AFI peaks, exhibits an additional peak shoulder at 22.6° and a minor peak at 12.1°, which are attributed to remnant carbon clusters (Sawant et al., 2017). At tested temperatures of 400°C and above, the above peaks disappear indicating that the remnant carbon content is significantly reduced or eliminated. Furthermore, such peaks do not exist in the untreated AlPO4-5 material either (Supplementary Figure 2). Thermal stability of a potential adsorbent is one of the key quality features for industrial application ensuring robustness upon thermal stresses that may occur during preparation as well as application. e.g., upon regeneration or other processing steps. The high temperature treatment applied here and the obtained stability of the AlPO4-5 adsorbents confirm their robustness and suitability for high temperature application. In order to explore the upper temperature stability limit, we also calcined the material at 850°C. There, we observed a structure collapse and transformation into a dense AlPO4-tridymite phase (Stoeger et al., 2012).
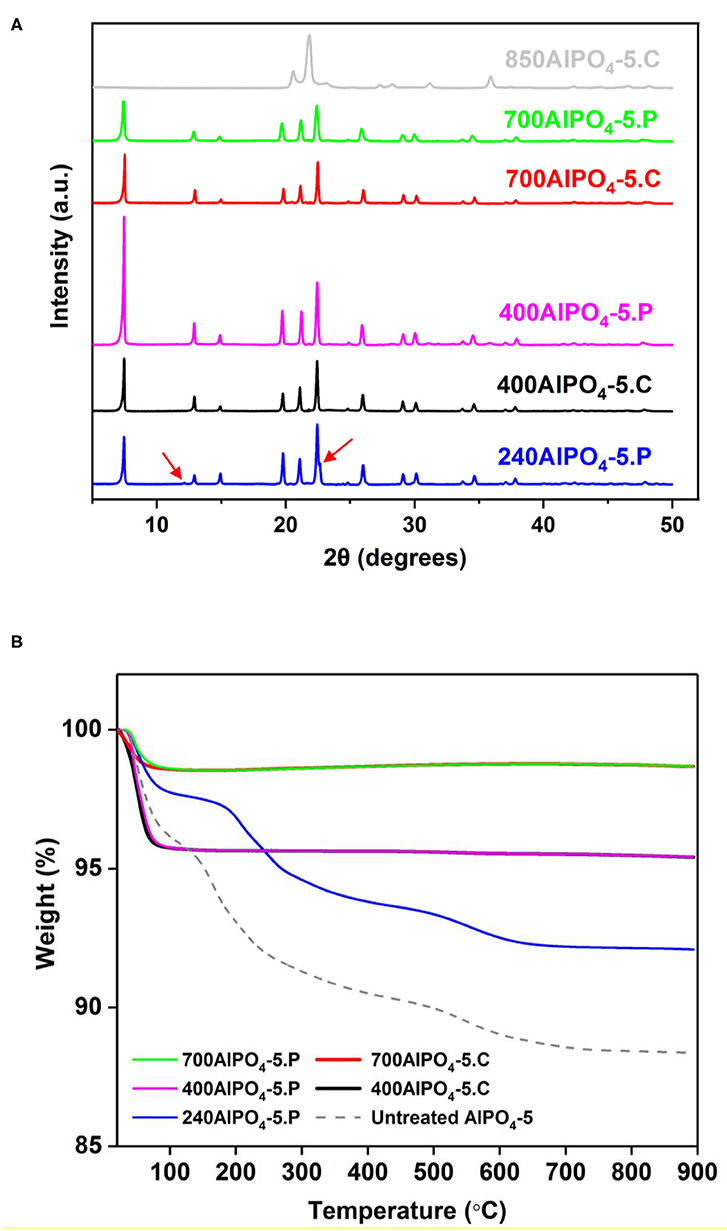
Figure 1. (A) XRD patterns and (B) TGA profiles of calcined and pyrolyzed AlPO4-5 adsorbents treated at different temperatures.
The carbon content of the thermally treated samples and their corresponding thermal behavior in comparison to untreated AlPO4-5 was further studied by TGA, which was performed under oxidative conditions (air). According to the obtained results (Figure 1B), the pyrolyzed and calcined adsorbents exhibit almost the same TGA profiles as revealed for the corresponding samples treated at 400 and 700°C Notably, the only significant weight loss experienced by both of the above sets of samples is the one at the low temperature range of up to 100°C, which is attributed to removal of adsorbed moisture. The absence of any noticeable transition due to amine decomposition indicates that the occluded amine molecules have been almost completely removed from the pores at these temperatures. In addition, the samples treated at 700°C exhibit a more hydrophobic behavior containing 1.3 wt% of moisture compared to the ones treated at 400°C that contain approximately 4.2 wt% of moisture (Table 1). This is attributed to the fact that polar functional groups on the AlPO4-5 surface, such as Al-OH and P-OH (Peri, 1971), are being compromised by the higher temperature treatment, thus suppressing hydrophilicity. The untreated AlPO4-5 adsorbent and the one pyrolyzed at 240°C exhibit two distinct weight loss transitions after the removal of the adsorbed water. The first transition is attributed to decomposition of the TEA molecules from the pores and occurs in the temperature range of 120–300°C for the untreated sample, while it starts at a higher temperature (165°C) for the pyrolyzed one. This temperature difference is due to the fact that portion of the amine has already been decomposed in the latter sample. The second transition occurs at a considerably higher temperature (500–650°C) and is attributed to the removal of trapped and possibly graphitized carbon species from the pores. As per Table 1, the total organic content in the untreated sample is 7.8 wt%, whereas that of the sample pyrolyzed at 240°C is 5.66 wt%. The existence of remnant carbon in the latter sample is in agreement to XRD evidence discussed above.
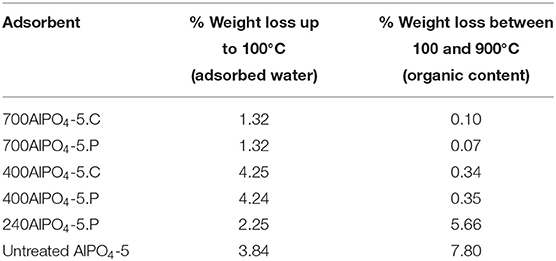
Table 1. Characteristic TGA transitions and corresponding weight loss percentages for the various calcined and pyrolyzed AlPO4-5 adsorbents.
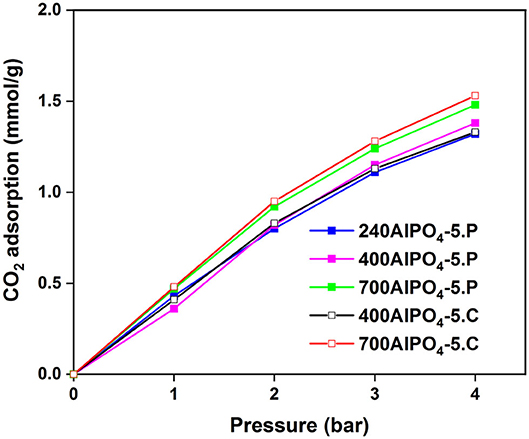
The effect of the calcination and pyrolysis treatment on the CO2 adsorption capacity is depicted in Figure 2 (data in Supplementary Table 1). The sample calcined at 700°C exhibits higher CO2 capacity (1.53 mmol/g at 4 bar) than the one calcined at 400°C. This observation is consistent throughout the pressure range examined (up to 4 bar) and indicates that maximizing porosity in the channels of the adsorbent is critical. The sample pyrolyzed at 700°C exhibits higher CO2 capacity compared to the other pyrolyzed samples throughout the pressure range of 0 to 4 bar, with a maximum capacity of 1.48 mmol/g at 4 bar. This is attributed to the total decomposition and removal of TEA from the framework, and the creation of clean pores in the zeolite to host the CO2 molecules. The remaining two isotherms corresponding to the lower pyrolysis temperature samples (240AlPO4-5.P and 400AlPO4-5.P) are of interest since an inversion in the CO2 adsorption capacity can be observed. Specifically, the sample which was treated at 240°C exhibits higher capacity at low pressures (up to 2 bar), whereas at higher pressures (2–4 bar) the sample treated at 400°C adsorbs more CO2. In addition, a noticeable observation with respect to low pressure CO2 capture application is that, up to 1 bar, the sample pyrolyzed at 240°C exhibits almost same capacity as the one pyrolyzed at the highest temperature tested, i.e., 700°C, which is also very close to the capacity observed for the calcined sample at 700°C. This behavior indicates that the carbon species remnants into the pores created by low temperature SDA pyrolysis interact efficiently with CO2 at low pressures, whereas capture capacity at higher pressures is more favored by the increased pore volume and carbon-free surface created upon higher temperature thermal treatment. Conclusively, if the AlPO4-5 adsorbents are to be used for low-pressure CO2 capture, high thermal activation is not required, as similar capture capacity can be achieved by pyrolysis treatment at significantly lower temperatures, e.g., 240°C, thus saving energy and safeguarding the thermal stability of the adsorbents. The relatively high capacity resulting from such pyrolysis-based activation treatment is attributed to enhanced interaction of CO2 at low pressures with carbon species that are remnant in the pores upon partial SDA decomposition.
Effect of Metal Substitution
Ion-substituted AlPO4-5 were prepared by incorporating Fe, Mg, Co, and Si into the framework of AlPO4-5. Ion substitution in AlPO4-5 takes place through various mechanisms depending on the substituting element, with divalent and trivalent ions substituting Al, whereas tetravalent ions such as silicon substitutes predominantly P at low Si content, whereas at higher Si ratios a pair of Si ions substitutes adjacent Al and P ions (Gaber et al., 2020). In the present set of experiments, a water/Al2O3 molar ratio of 100:1 and a metal/Al2O3 ratio of 5:100 were used. The XRD patterns of the resulting MeAPOs (Figure 3A) are in accordance with the AFI structure possessing all the AFI peaks, including the characteristic ones at 2θ of approximately 20, 22, and 23° that correspond to the reflections from the (210) (002) and (211) crystallographic planes, respectively (Karanikolos et al., 2008; Stoeger et al., 2012; Basina et al., 2018). Furthermore, the materials exhibit high crystallinity as no broad peaks or shoulders and no evidence of secondary crystalline phases/impurities were noticed. A closer look at the XRD patterns reveals that some peaks of the ion substituted AlPOs are not perfectly aligned with those of the AlPO4-5. Indeed, Figure 3B shows the three characteristic AFI peaks extending between 19 and 23°, which are attributed to the crystallographic (210), (002) and (211) planes where minor shifts are evident. These shifts are attributed to the ion substitution and incorporation into the framework since heteroatoms with different ionic radii are inserted into the lattice substituting Al and/or P. The ionic radius of alumina is 0.53 Å with all substituting metals having larger ionic radii, i.e., Fe: 0.645 Å, Mg: 0.72 Å, and Co: 0.745 Å. Silica possesses an ionic radius is 0.4 Å and has the ability to substitute phosphorus, which has an ionic radius 0.38 Å (SM2 mechanism). Notably, Fe exhibits the biggest expansion along the c-dimension. These shifts confirm the successful substitution of the metals into the AlPO framework. Analogous results were reported in our previous work, where AlPO4-5 metal substitution resulted in change of lattice parameters (Gaber et al., 2020).
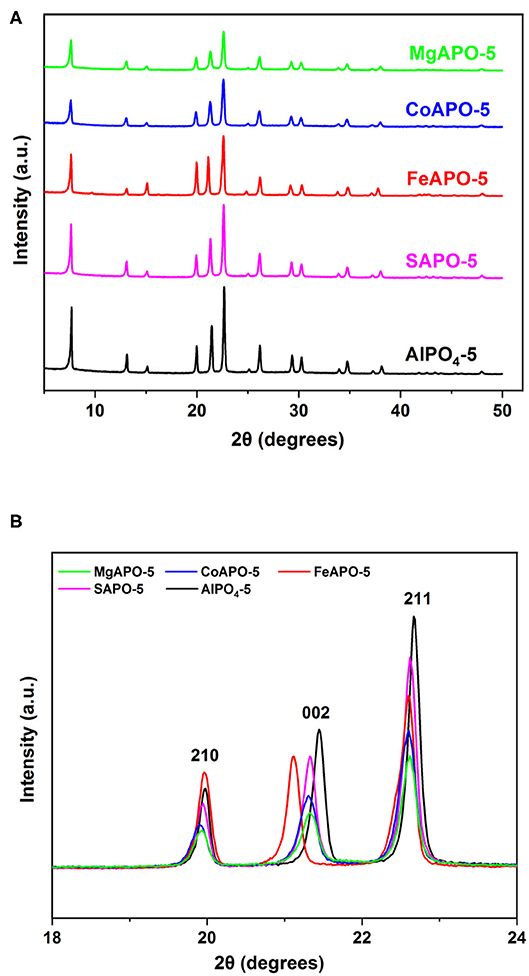
Figure 3. (A) XRD patterns of MeAPOs with Me/Al2O3 molar ratio in the synthesis mixture of 5:100, and (B) closer view of the AFI characteristic peaks between 19 and 23°.
SEM images of calcined AlPO4-5 and MeAPO-5 are depicted in Figure 4. The materials are comprised of crystalline particles, thus confirming the XRD findings. All images correspond to adsorbents generated from dense reaction mixtures (H2O/Al2O3 molar ratio of 100), except Figure 4c that corresponds to dilute reaction mixture (H2O/Al2O3 molar ratio of 400) and the crystals exhibit columnar morphology. According to the low magnification images, the particles of the materials with H2O/Al2O3 molar ratio of 100 have spherical shape with uniform sizes that range between 20 and 30 μm. In high magnification, it is evident that the spherical particles are agglomerates of flat rectangular-like small crystals. In FeAPO-5 (Figures 4d,f), an analogous morphology is observed, whereas it is evident that the crystal aggregates tend to acquire a more hexagonal shape (Figure 4f) following the AFI crystal structure. This reveals that the particles in this adsorbent have been formed not by simple physical aggregation of the small individual crystallites, but rather a considerable degree of interaction and coalescence have taken place upon crystal growth.

Figure 4. SEM images of (a,b) 100AlPO4-5, (c) 400AlPO4-5, (d–f) FeAPO-5, (g,h) MgAPO-5, (i,j) CoAPO-5, and (k,l) SAPO-5 adsorbents.
The MgAPO-5 crystal aggregates (Figures 4g,h) exhibit spherical shape with an average particle diameter of 20 μm. The particle size of MgAPO-5 is smaller than that of FeAPO-5, whereas the aggregates seem to have a more perfect spherical shape compared to the hexagonal configuration observed in FeAPO-5. This reveals that interaction and coalescence between the individual small crystallites in MgAPO-5 is lower compared to that in FeAPO-5. CoAPO-5 (Figures 4i,j) also mainly consists of spherical particles, but they are quite heterogeneous since some of them have holes at their axis. The majority of SAPO-5 crystals are spherical, but some larger, irregularly shaped aggregates have also formed by further merging of the spherical particles, as shown in Figures 4k,l.
The morphology of AlPO4-5 prepared at high dilution rate (H2O/Al2O3 molar ratio in the synthesis mixture of 400, Figure 4c) reveals mainly columnar, hexagonal monocrystals (Cheung et al., 2012; Gaber et al., 2020). This is attributed to the fact that dilution of the reaction gel decreases the nucleation rate (Du et al., 1997), and favors preferential growth along the c-axis of the crystals. Indeed, the spheres formed in the samples using dense synthesis mixtures (H2O/Al2O3 molar ratio of 100) are comprised of smaller rectangular-like crystals that are tightly interconnected. The progress of crystallization controls the size of the spherical aggregates and prove to be rather homogeneous. FeAPO-5 particles tend to acquire a hexagonal shape in accordance to the AFI morphology compared to the rest of materials grown using dense reaction mixtures, thus indicating a closer interaction and coalescence among the individual flat-like crystallites that comprise each aggregate. This can be correlated to the XRD observations (Figure 3B), where FeAPO-5 was shown to exhibit the largest shift in the (002) peak, which may be also partially associated to the interface Fe ions shared among coalesced crystallites. Increasing the dilution slows down the crystallization rate, thus forming hexagonal, rod-like shape crystals following preferential growth along the c-axis (Iwasaki et al., 2003; Karanikolos et al., 2008). However, due to slow crystallization, it is possible that these samples may also possess some amorphous areas (Utchariyajit and Wongkasemjit, 2008) and low crystallinity regions.
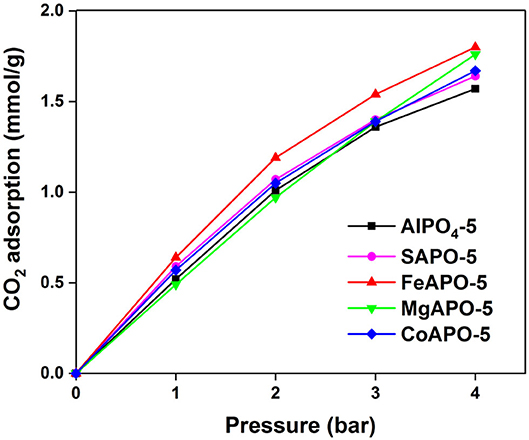
The CO2 adsorption results of the metal substituted AlPOs and the parent AlPO4-5 are shown in Figure 5 (data tabulated in Supplementary Table 2). Overall, MeAPO-5 samples exhibit higher capacity than the parent AlPO4-5 indicating that metal substitution promotes CO2 adsorption. Among all MeAPO-5 samples, FeAPO-5 exhibits the highest capacity throughout the pressure range tested, reaching a maximum value of 1.8 mmol/g at 4 bar, which is 15% higher than that of the parent AlPO4-5 at the same conditions, owing to the substitution of Al3+ with Fe3+ in the lattice. At the lower pressure range of up to 3 bar, the Fe- and Co-substituted analogs exhibit higher capacity than the Mg-substituted one due to the fact that the presence of transition metal elements (Fe and Co) in the lattice tend to enhance the affinity between CO2 with the inorganic framework compared to alkali earth metals (Mg) and weak metals (Al) (Yu et al., 2018).
The CO2 capacity of MgAPO-5 is the lowest at low CO2 partial pressures even when compared to the parent AlPO4-5 adsorbent, whereas at pressures higher than 2.5 bar it displays an increase reaching at 4 bar at an almost same value as that of FeAPO-5. Strong ionic character and bond length in this case (Mg-O 1.969 Å) could influence CO2 sorption (Caskey et al., 2008; Yazaydin et al., 2009), with a possible clustering effect of CO2 molecules in the vicinity of Mg within the pores taking place at higher pressures. In addition, diffusion limitations within the pores as well as in the interstitial spaces among the crystallites in each particle agglomerate could be overcome as pressure increases. CoAPO-5 and SAPO-5 contain acidic sites within their framework due to charge imbalance upon ion substitution. Their sorption capacity is slightly higher compared to the parent AlPO4-5 adsorbent (by 6% and 4%, respectively). Analogous results were reported using in situ IR spectroscopy studies on SAPO-56 adsorbents possessing a high concentration of acid sites (Cheung et al., 2012). Effect of ion substitution on the lattice parameters may play also a role in CO2 adsorption. The substituting metals have two choices in AlPO4-5, i.e., to substitute Al and P positions. In general, Si will preferentially substitute for P, and divalent and trivalent ions will replace Al (Wilson et al., 1982). Substitution by transition metals with higher ionic radius (Fe, Mg, and Co) will generally increase the value of lattice parameters than low ionic radius elements such as Si (Kaneko and Rodríguez-Reinoso, 2019), thus enhancing the CO2 adsorption capacity.
Effect of Metal Content
The enhanced CO2 adsorption capacity of FeAPO-5 compared to all other AlPO samples tested led us to further investigate this material by varying the molar composition of iron in the precursor mixture. An initial molar ratio of Fe/Al2O3 of 5:100 was used as a basis, and a set of materials with three more Fe/Al2O3 molar ratios, namely, 2.5:100, 7.5:100, and 10:100, were additionally synthesized and studied.
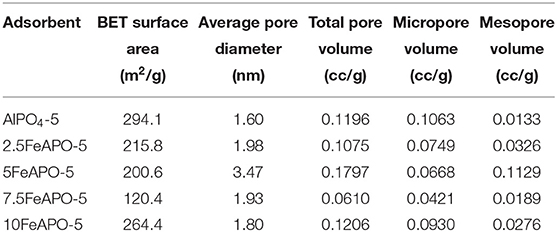
The liquid N2 adsorption–desorption isotherms of the FeAPO-5 adsorbents are shown in Figure 6A, while the resulting physical and pore properties estimated from BET analysis, BJH, and HK methods are presented in Table 2. All adsorbents exhibit a steep nitrogen uptake at a low relative pressure (P/Po) confirming their microporous nature. The adsorbents display a type-IV isotherm with hysteresis loops extending across relative pressures of 0.45 to 0.9, which confirm also the existence of mesopores. Both micropore and mesopore size distributions are shown in insets i and ii, respectively. Among the tested samples, the steepest increase at high relative pressures (P/Po > 0.8) and the most extensive hysteresis loop was observed for 5FeAPO-5, which are indicative of an extended mesoporous network (Figure 6B). According to the micropore size distribution, all-metal substituted samples possess a narrow peak centered at ~0.7 nm, which confirms their AFI structure (Karanikolos et al., 2008). From Table 2, the BET surface area of FeAPO-5 adsorbents varies from 120 to 264 m2/g, the pore volume from 0.06 to 0.18 cc/g, and the average pore diameter from 1.8 to 3.5 nm. The relatively wide ranges observed reveal the considerable affect that the metal incorporation induces to the physical properties of the adsorbents.
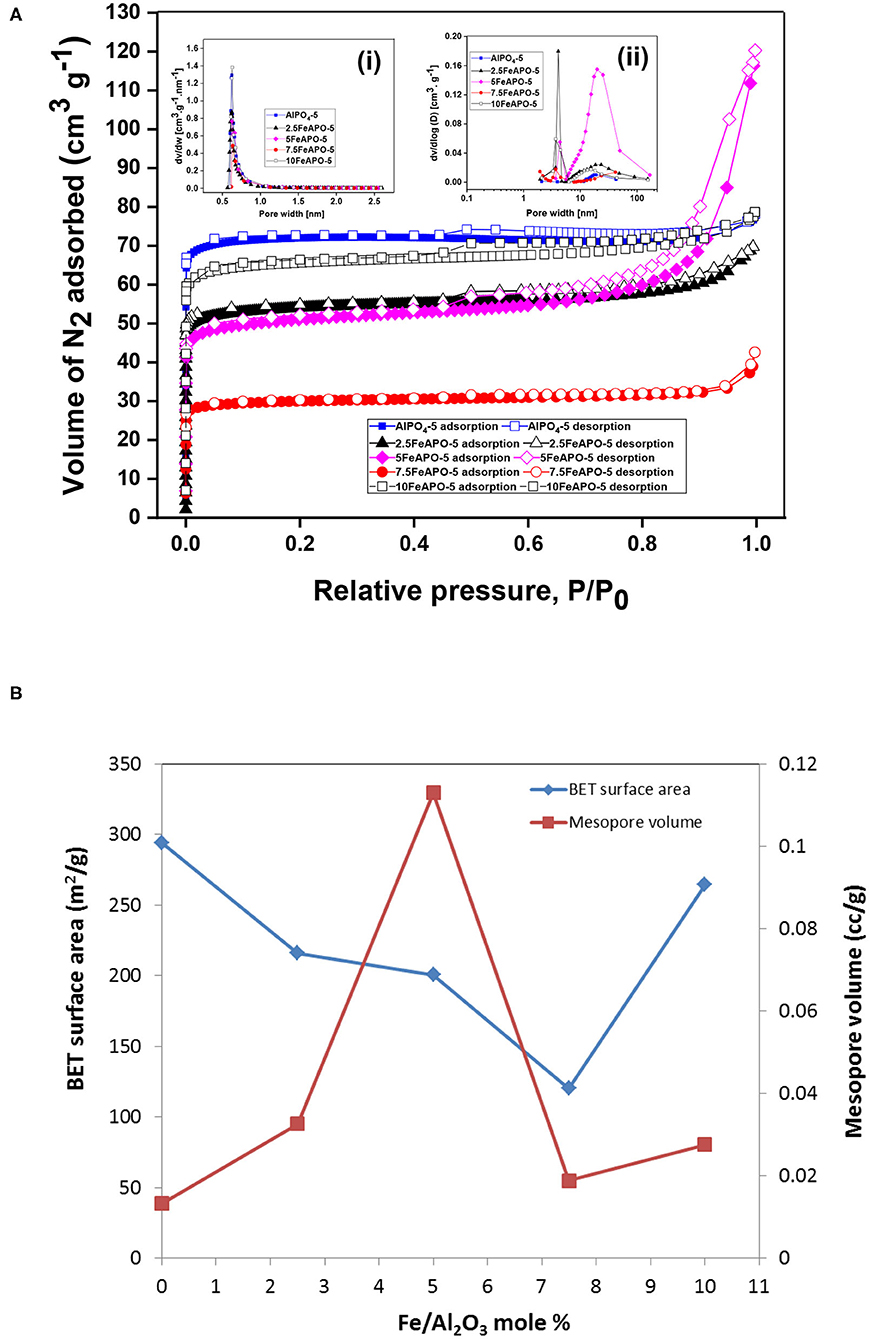
Figure 6. (A) Liquid N2 adsorption–desorption isotherms of FeAPOs. Inset (i): Micropore size distribution determined by the HK method. Inset (ii) BJH-derived mesopore size distribution. (B) BET surface area and mesopore volume as a function of Fe/Al2O3 molar content (with respect to %Al2O3) in the synthesis mixture.
To shed more light on the structural effects of the metal content, we also performed XRD, FTIR, and UV-Vis DRS analysis of the FeAPO-5 adsorbents. According to the XRD patterns (Figure 7A), the structural integrity of the AFI framework is retained for the whole range of Fe/Al2O3 % molar ratios tested (2.5–10), with no evidence of metal incorporation effects in the AFI crystallinity or appearance of secondary phases/impurities. FTIR spectra are shown in Figure 7B. The band at 3,535 cm−1 is ascribed to –OH functional groups, whereas the bands at 1,219, 716, and 615 cm−1 are ascribed to the asymmetric and symmetric stretching vibrations of the Al-O-P units (Zhang et al., 2020). Comparing the FeAPO-5 samples with the non-substituted AlPO4-5, the band at 562 cm−1 due to Al-O or P-O bending modes of AlPO4-5 framework is significantly suppressed upon Fe incorporation, whereas the band at 1,112 cm−1, which corresponds to stretching vibration of Al–O in combination with P–O (Chen and Jehng, 2003), is shifted to lower wavenumber (cm−1) values. Comparison among the various FeAPO-5 samples does not reveal any noticeable differences in FTIR bands as to differentiate among the Fe loadings and/or possible Fe segregation. UV-Vis DRS spectra of the Fe-substituted adsorbents are shown in Figure 7C. A dominant peak centered at 263 nm is evident for all FeAPO-5 samples with a shoulder at 235 nm attributed to the ligand to metal charge transfer of Fe3+ in [FeO4]− tetrahedral geometry (Mohapatra et al., 2002). The intensity of the above band for the unsubstituted AlPO4-5 is negligible compared to the iron containing samples (Feng et al., 2016). Notably, for the sample with the highest metal content (10FeAPO-5) the above peak is shifted to higher wavelengths, which is indicative of increased amount of octahedral complexes in extra-framework positions, and the distribution state for the Fe species corresponding to 280 nm in particular can be isolated or clustered (Feng et al., 2016). The broad band between 400 and 500 nm is due to Fe d–d transitions and is also indicative of clustering of iron species (Wei et al., 2008). Notably, the intensity of the above band for the 5FeAPO-5 sample is minimal.
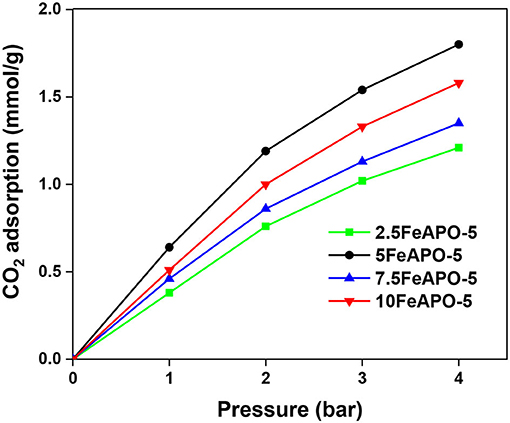
The FeAPO-5 adsorbents exhibit both micropores with an average diameter of ~0.7 nm, which is bigger than the kinetic diameter of CO2 (0.33 nm), thus posing no kinetic restriction for CO2 adsorption, as well as a relatively extended mesoporous network (Figure 6A). In such configuration, at low partial pressure of CO2, monolayer adsorption and adsorption on the micropores are anticipated to occur first, whereas mesopores are filled as pressure increases and adsorbate–adsorbate interactions are being enhanced. The CO2 adsorption capacity of the FeAPO-5 adsorbents with different metal concentrations are shown in Figure 8 (data also in Supplementary Table 3). According to Figure 6B, the BET surface area decreases with Fe content until a Fe/Al molar ratio of 7.5:100 and then it increases again. The mesopore volume follows an opposite trend, i.e., it increases with Fe content, exhibits a maximum at Fe/Al ratio of 5:100, and then it decreases. As shown by UV-Vis DRS analysis above, for high metal concentration in the synthesis mixture, a portion of the available Fe forms clusters, and it is not incorporated into the AFI framework as tetrahedrally coordinated ions by substituting Al3+. The existence of amorphous Fe3+ phase for high metal loading has been confirmed also before via various spectroscopic and chemical probe methods (Das et al., 1992). Consequently, the CO2 adsorption capacity does not exhibit a regular trend as Fe content increases, rather it exhibits a maximum for the sample corresponding to Fe/Al2O3 ratio of 5:100, which exhibits the higher volume of mesopores and the minimum portion of extra-framework or clustered iron as revealed by the UV-Vis analysis. Indeed, the adsorption capacity of 5FeAPO-5 at low pressure (1 bar) is 0.64 mmol/g and at high pressure (4 bar) it becomes 1.8 mmol/g, corresponding to an incremental increase of 1.16 mmol/g from the above low to high pressure values, which is the highest increment among the FeAPO-5 adsorbents tested. This is attributed to the contribution of mesopores in the adsorption capacity which becomes dominant at higher pressures. Conclusively, Fe incorporation enhances the mesoporosity despite the fact that it decreases the surface area. The latter is more important for relatively low metal content causing a reduction in CO2 capacity compared to pure AlPO4-5, yet as metal content increases, the mesoporosity formation becomes dominant and brings the capacity to higher values than that of the pure AlPO4-5 at same conditions. However, for high metal loadings, some Fe prefers to cluster upon growth forming amorphous Fe3+ phases and/or extra-framework species thus associated to reduced mesoporosity and lower CO2 capacity.
Effect of Reaction Mixture Dilution and Associated AlPO4-5 Morphology
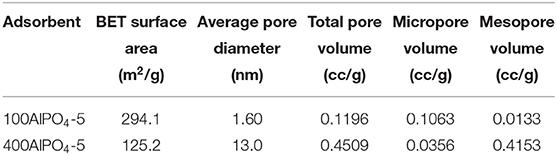
In our previous work we have shown that a critical parameter that strongly affects crystal morphology upon AlPO4-5 growth is the water content in the synthesis mixture (Karanikolos et al., 2008). Specifically, it was demonstrated that dense reaction mixtures favor growth perpendicular to the AFI channels, i.e., along the a-b directions, while diluted reaction mixtures induce preferential growth along the c-direction, i.e., parallel to the channels. Accordingly, we employed here two different dilutions in the reaction mixture, namely, H2O/Al2O3 molar ratio of 100 (sample 100AlPO4-5) and 400 (sample 400AlPO4-5), which resulted in spherical agglomerates of small flake-like crystallites, and columnar monocrystals, respectively (Figures 4A–C). From XRD analysis (Figure 9A), it is evident that both samples possess the AFI structure, yet the lower-dilution sample displays a higher crystallinity when compared to the high-dilution one.
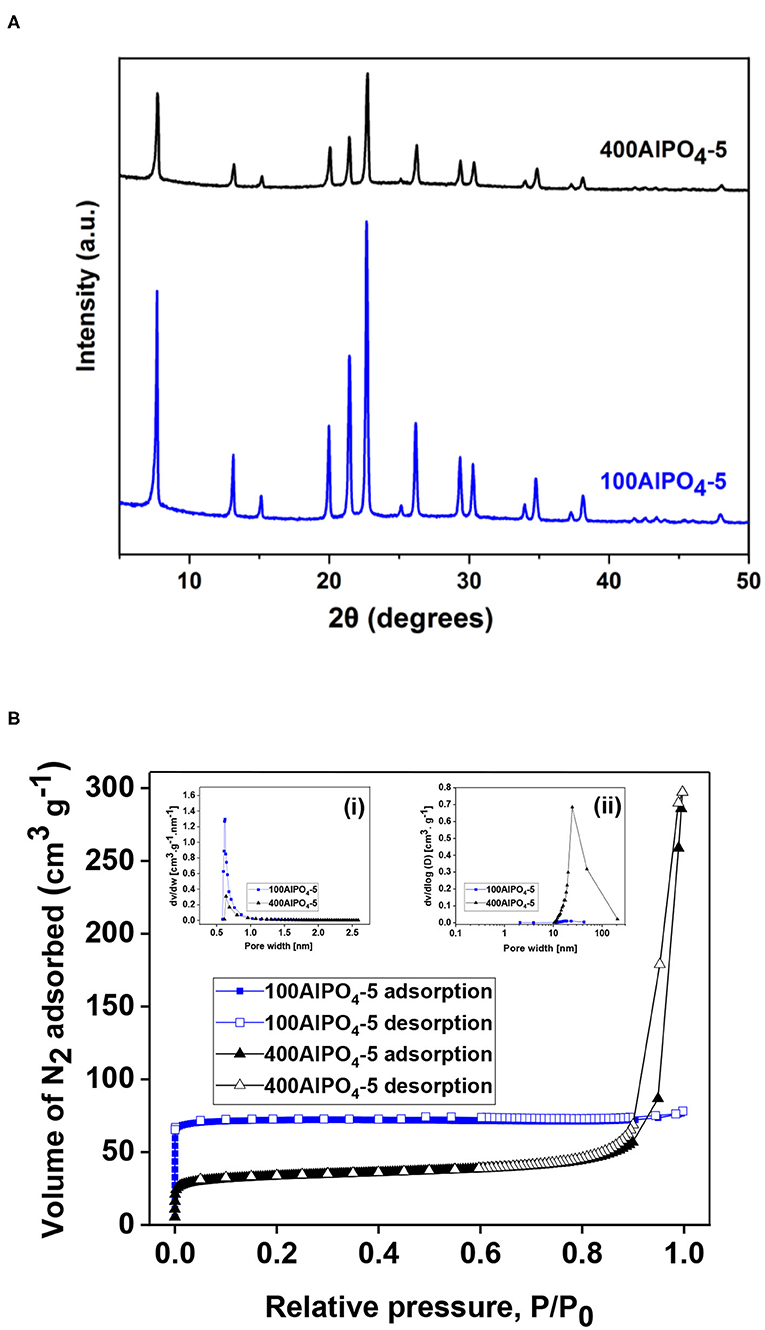
Figure 9. (A) XRD patterns for AlPO4-5 crystals corresponding to two different dilution rates in the reaction mixture, i.e., H2O/Al2O3 molar ratio of 100 and 400 (100AlPO4-5 and 400AlPO4-5). (B) N2 adsorption isotherms of 100AlPO4-5 and 400AlPO4-5 at 77K, inset (i): micropore size distribution determined by the HK method, inset (ii): BJH mesopore size distribution.
The liquid N2 adsorption isotherms and pore size distributions of the 100AlPO4-5 and 400AlPO4-5 adsorbents are shown in Figure 9B, and the associated physical and porosity properties are presented in Table 3. It is evident that diluted reaction mixtures yield AlPO4-5 with lower surface area and micropore volume, yet enhanced mesoporosity. The shape of the isotherms also confirms this observation, as the isotherm of 100AlPO4-5 approaches that of type-I revealing a strongly microporous nature with negligible contribution from larger pores, whereas that corresponding to 400AlPO4-5 is a type-IV isotherm with a steep rise in adsorption at P/P0 > 0.85 and an enhanced hysteresis loop revealing capillary condensation in mesopores. Indeed, the surface area drops from 294 to 125 m2/g while the mesopore volume increases from 0.0133 to 0.4153 cc/g for the 100AlPO4-5 and 400AlPO4-5 adsorbents, respectively. The micropore size distribution (Figure 9B, inset ii) is narrow averaging at ~ 0.7 nm for both materials, in accordance to AFI structure, whereas the portion of larger pores is relatively low for 100AlPO4-5, and becomes significant for 400AlPO4-5, for which an average mesopore width of 25 nm is evidenced from Figure 9B, inset.
The CO2 adsorption isotherms and associated capacities for the AlPO4-5 adsorbents corresponding to low and high dilutions of the reaction mixture are shown in Figure 10. It is evident that dense reaction mixtures (100AlPO4-5) yield adsorbents that exhibit higher capacity compared to diluted ones (400AlPO4-5). Indeed, 100AlPO4-5 exhibits an adsorption capacity of 1.57 mmol CO2/g at 4 bar and 25°C, which is almost double than that of 400AlPO4-5. As discussed earlier for the Fe-substituted adsorbents, a high mesopore volume is favorable for enhancing CO2 capacity. Nevertheless, this does not seem to be the case for the non-substituted materials where substituting heteroatoms do not exist. Here, the effect of BET surface area reduction for the adsorbent corresponding to high dilution rate of the reaction mixture is more dominant than the mesoporosity formation, thus causing a decrease in CO2 capacity compared to the adsorbent originated from dense mixture. In addition, a high average mesopore diameter (25 nm in the case of 400AlPO4-5) might decrease the interaction potential, affinity between CO2-CO2, and retention upon multilayer adsorption.
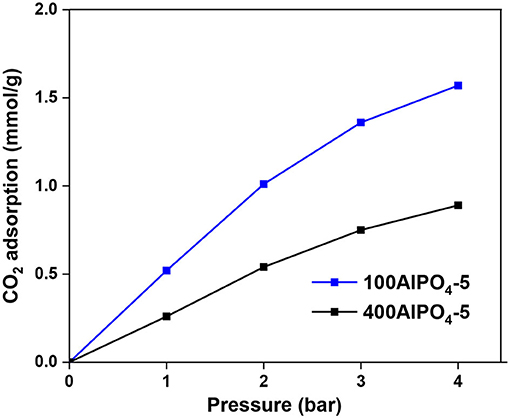
Figure 10. CO2 adsorption of AlPO4-5 adsorbents corresponding to two different reaction mixture dilution rates and associated crystal morphologies at 25°C.
Isosteric Heat of Adsorption
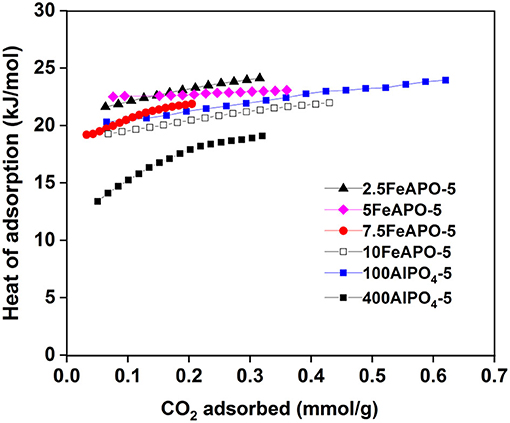
The isosteric heat of adsorption concerns the amount of energy that is released when CO2 adsorbs onto the adsorbent surface at a fixed coverage. Consequently, at least the same amount of energy must be added in order for the CO2 to be desorbed from the adsorbent upon regeneration. In addition, the isosteric heat of adsorption values provide a quantifiable indicator of the affinity of an adsorbate molecule toward the adsorbent surface. CO2 adsorption isotherms were collected at three different temperatures in order to calculate the heat of adsorption of the AlPO adsorbents at different CO2 loadings. The isosteric heat of adsorption values were determined based on the Clausius–Clapeyron equation using the obtained isotherm data, and the results are depicted in Figure 11.
The heat of adsorption values for all the studied adsorbents are lower than 25 kJ/mol throughout the tested coverages, which indicates that the mechanism of CO2 adsorption in these materials is physisorption, in line to the reported literature (Simmons et al., 2011). As such, they hold a high potential for PSA-based capture application as desorption can easily be enabled by pressure swing without the need of thermal regeneration. According to the obtained profiles, the heat of adsorption increases as the CO2 loading increases for all the studied adsorbents, implying that, as coverage increases, adsorbate–adsorbate (CO2-CO2) interactions are stronger than adsorbate–adsorbent interactions on CO2-philic surface sites. Concerning the Fe-substituted analogs in particular, the ones corresponding to relatively low Fe/Al2O3 molar ratio in the synthesis mixture (2.5:100 and 5:100) exhibit the highest heat of adsorption values compared to the rest of tested adsorbents. This indicates that the incorporated metal enhances the physical binding, a fact that is more pronounced for 5FeAPO-5 at low coverage indicating a stronger CO2 interaction on the adsorbent surface upon monolayer formation. Notably, the above mentioned adsorbent exhibited the highest uptake compared to the rest of the ion-substituted materials tested as well as to the pure AlPO4-5 (Figures 5, 8). Furthermore, the heat of adsorption curve of 5FeAPO-5 is almost flat, which is attributed to high crystallinity and homogenous surface with a uniform distribution of the Fe heteroatoms into the framework lattice that enhance binding. Notably, this adsorbent contains the lowest portion of extra-framework or clustered iron among all FeAPO-5 materials tested (Figure 7C). As Fe content in the synthesis mixture increases the heat of adsorption, and thus the CO2 binding strength with the adsorbent surface decreases, a result that is in agreement to the observed reduced CO2 adsorption capacity for 7.5FeAPO-5 and 10FeAPO-5, which is attributed to the tendency of the readily available Fe ions at these high metal concentrations to cluster in the synthesis mixture and yield formation of amorphous Fe3+ phases upon crystal growth (Das et al., 1992), as also discussed above (Figure 7C), and/or remain as extra-framework species partially blocking active surface sites.
The low value of heat of adsorption for the 400AlPO4-5 adsorbent, which corresponds to diluted reaction mixture, indicates that the morphology and textural and surface properties of this material, including the large pore width, large mesopore volume, and rod-like crystal morphology, promote a very weak binding with the surface, which is in agreement to the low CO2 adsorption capacity obtained for this material. In addition, the heat of adsorption curve as a function of coverage for this adsorbent exhibits the largest slope among all tested adsorbents for the low coverage range (up to 0.22 mm/g). This is indicative of the initially weak interaction of CO2 with the surface and during monolayer adsorption, which becomes stronger as coverage increases due to more dominant adsorbate–adsorbate interactions. At higher coverages (>0.22 mmol/g), the slope becomes lower and almost equalizes with that of the other tested adsorbents.
CO2 Adsorption Kinetics
Understanding of the adsorption kinetics of an adsorbent is vital in designing PSA systems (Loganathan et al., 2014). Various adsorption kinetic models have been employed, among which, the pseudo–first-order kinetics or Lagergren model (Liu et al., 2014) is widely used for CO2 adsorption. The pseudo–first-order equation models a reversible interaction between adsorbent and adsorbate. This is suitable for describing the physical adsorption of CO2 on solid adsorbents (Serna-Guerrero and Sayari, 2010; Loganathan et al., 2014). The pseudo–first-order model is governed by Equation (1):
where qe and qt (mg/g) represent the amount of CO2 adsorbed at equilibrium and at a given time “t”, respectively, and k (min−1) is the first order rate constant. The relevant boundary conditions are BC1: t = 0, qt = 0 and BC2: t = ∞, qt = qe. Applying the above boundary conditions to Equation (1) while solving the aforementioned differential equation leads to Equation (2).
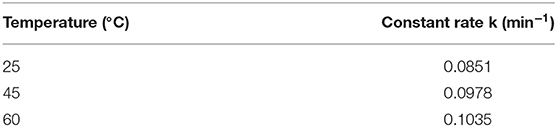
The higher CO2 adsorption capacity and rate of the 100AlPO4-5 adsorbent compared to the 400AlPO4-5 analog, as confirmed by both equilibrium data at various pressures (Figure 10), and kinetic data up to 4 bar (Supplementary Figure 3) led us to further investigate the kinetic behavior of this adsorbent by conducting adsorption experiments at three different temperatures, i.e., 25, 45, and 60°C up to a pressure of 4 bar (Supplementary Table 5 and Supplementary Figure 4). The CO2 adsorption capacity decreases as temperature increases confirming the physisorption mechanism. In order to calculate the adsorption rate constant k for the three different temperatures, the adsorption data at the different temperatures were used to plot log (qe – qt) vs. t/2.303 (Supplementary Figure 5). The resulting linear fit slope is the adsorption rate constant (k), and the y-intercept is log qe (Liu and Shen, 2008; Liu et al., 2014). The adsorption rate constant increases as temperature increases (Table 4) yet with relatively slight differences indicating that the adsorption of CO2 on AlPO4-5 is rather thermodynamically limited. The equilibrium adsorption capacity (qe) was also calculated from the pseudo–first-order model and compared with the experimentally measured one (Supplementary Table 5). The model predicted the equilibrium adsorption capacity with an average relative error of 1.8–6.5%, and the value of the correlation coefficient (R2) of the model is around 0.98. Thus, the pseudo–first-order model proves to be qualitatively and quantitatively suitable for modeling of CO2 adsorption kinetics of the studied AlPO4-5 adsorbents as shown Figure 12A.
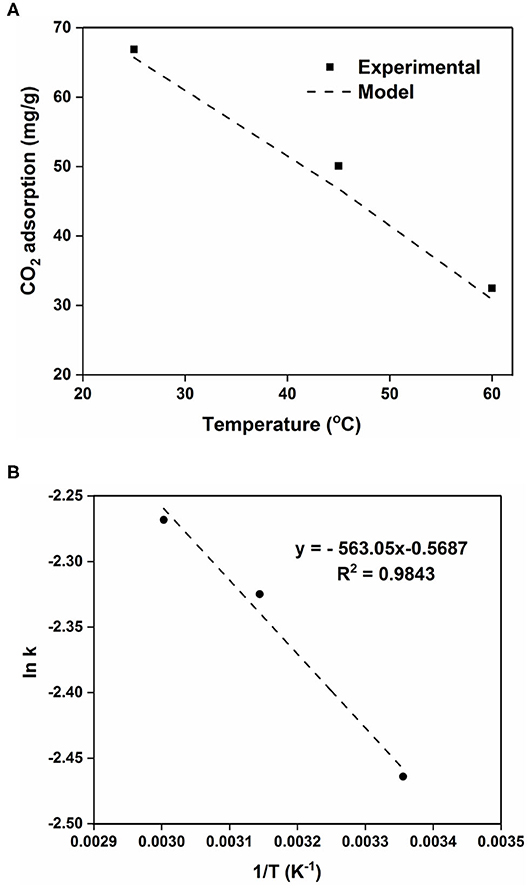
Figure 12. (A) Comparison of experimental data vs. model fitting data. (B) Arrhenius plot for the kinetic rate constant obtained by the linear Lagergren model for the 100AlPO4-5 adsorbent.
The temperature dependence of rate constant (k) can be described by the Arrhenius equation (Equation 3), where A is the pre-exponential factor, E is the activation energy, R is the universal gas constant, and T is the temperature in absolute units.
The plot of ln(k) vs. 1/T (Figure 12B) exhibits a linear profile, as anticipated from the linearized form of the Arrhenius equation. From the obtained slope, the activation energy for the CO2 adsorption on 100AlPO4-5 at 4 bar was calculated to be 4.7 kJ/mol. The obtained activation energy value is in close agreement to the reported values of CO2 adsorption on activated carbon and Zeolite 13X, where activation energies of 3.9 and 4.8 kJ/mol, respectively, were estimated up to CO2 pressure of 3 bar (Zhang et al., 2010).
Conclusions
Effects of metal substitution, synthesis mixture composition and associated morphology manipulation, and activation procedure on AlPO4-5 were studied for CO2 adsorption. Activation by calcination in air at high temperature was found to completely open up the pores resulting in higher CO2 capacity compared to calcination at lower temperatures. Yet, upon activation by pyrolysis in inert atmosphere, low temperature (240°C) treatment enhanced CO2 interaction with the surface at low pressures (up to 1 bar) due to the existence of remnant carbon species in the pores from the partial decomposition of the SDA. Ion substitution by Fe, Mg, Co, and Si induced changes in lattice parameters and morphology, whereas the Fe-substituted adsorbents exhibited the highest capacity compared to the rest of metal-substituted analogs. Parametric variation of the Fe content in the synthesis mixture revealed that, at relatively low metal concentrations, mesopore volume increases and microporosity and BET surface area decrease with metal content. At these conditions, the strong interaction of CO2 with the framework Fe and the adsorbate–adsorbate interactions in the formed mesopores increased the CO2 capacity. For high metal concentrations, some Fe prefers to cluster upon growth forming amorphous Fe3+ phases and/or extra-framework species, thus resulting in reduced mesoporosity and lower CO2 capacity. Dense reaction mixtures yielded AlPO4-5 consisting of spherical agglomerates of 2D-like crystallites that were almost exclusively microporous in nature, whereas diluted reaction mixtures resulted in crystals of columnar morphology exhibiting significant mesoporosity. In contrast to the metal-substituted analogs and thus in the absence of metal-CO2 interactions, the microporosity and high surface area of the AlPO4-5 adsorbents corresponding to dense reaction mixtures were dominant factors compared to the enhanced mesoporosity of the ones grown from diluted mixtures, thus yielding higher CO2 capacity for the former materials.
The isosteric heat of adsorption values for all the studied adsorbents were lower than 25 kJ/mol indicating that the mechanism of CO2 adsorption is physisorption, which is suitable for PSA application. The Fe-substituted analog corresponding to a Fe/Al2O3 molar ratio in the reaction mixture of 5:100 exhibited the highest heat of adsorption at low coverage, indicating affinity and stronger physical binding of CO2 with the framework-incorporated Fe. Notably, this adsorbent exhibited the lowest portion of extra-framework or clustered iron. Kinetic analysis revealed that a pseudo–first-order model could describe well the CO2 adsorption kinetics of the AlPO4-5 adsorbents, with an activation energy of 4.7 kJ/mol at 4 bar and adsorption rate constants increasing with temperature, yet with rather slight differences. AlPO4-5–based adsorbents, though they exhibit rather moderate capacities, they are robust, thermally and chemically stable materials, they exhibit low heat of adsorption, thus potential for PSA application, and limited hydrophilicity compared to classical zeolites that makes them important for CO2 capture from wet streams. The parametric investigation performed in this work sheds light on main optimization factors and paves the way for further studies toward implementation at industrial scale.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
AP performed the synthesis/growth, characterization of the adsorbents, and drafted part of the manuscript. KR performed the CO2 evaluation and drafted part of the manuscript. DK edited the manuscript and contributed in the supervision of AP. DR edited the manuscript and contributed in the supervision of the activities. YA contributed in the supervision of the activities, provided feedback and ideas, contributed in the design of the experiments, and edited the manuscript. GK set up and designed the project, attracted the funding, supervised the activities, and drafted/edited the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge support by the R&D division of the Abu Dhabi National Oil Company (ADNOC, project RDProj.018-GP) and Khalifa University (Award No. CIRA-2020-093). We thank Ms. Abeer Ali Nasser Al Yafeai, Ms. Tharalekshmy Anjana, and Ms. Dina Ali Gaber for assistance with SEM, XRD, and UV-Vis characterizations, respectively.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2020.568669/full#supplementary-material
References
Basina, G., AlShami, D., Polychronopoulou, K., Tzitzios, V., Balasubramanian, V., Dawaymeh, F., et al. (2018). Hierarchical AlPO4-5 and SAPO-5 microporous molecular sieves with mesoporous connectivity for water sorption applications. Surface Coat. Technol. 353, 378–386. doi: 10.1016/j.surfcoat.2018.08.083
Carmine, C. (2012). The fascinating story of porous materials. Curr. Phys. Chem. 2, 126–135. doi: 10.2174/1877946811202020126
Caskey, S. R., Wong-Foy, A. G., and Matzger, A. J. (2008). Dramatic tuning of carbon dioxide uptake via metal substitution in a coordination polymer with cylindrical pores. J. Am. Chem. Soc. 130, 10870–10871. doi: 10.1021/ja8036096
Chen, C.-M., and Jehng, J.-M. (2003). Effect of synthesis pH and H2O molar ratio on the structure and morphology of aluminum phosphate (AlPO-5) molecular sieves. Catal. Lett. 85, 73–80. doi: 10.1023/A:1022120824681
Cheung, O., Liu, Q., Bacsik, Z., and Hedin, N. (2012). Silicoaluminophosphates as CO2 sorbents. Microp. Mesop. Mater. 156, 90–96. doi: 10.1016/j.micromeso.2012.02.003
Choi, S., Drese, J. H., and Jones, C. W. (2009). Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem 2, 796–854. doi: 10.1002/cssc.200900036
Chou, C. T., and Chen, C. Y. (2004). Carbon dioxide recovery by vacuum swing adsorption. Sep. Purif. Technol. 39, 51–65. doi: 10.1016/j.seppur.2003.12.009
Chue, K. T., Kim, J. N., Yoo, Y. J., Cho, S. H., and Yang, R. T. (1995). Comparison of activated carbon and zeolite 13X for CO2 recovery from flue gas by pressure swing adsorption. Indu. Eng. Chem. Res. 34, 591–598. doi: 10.1021/ie00041a020
Corma, A. (2003). State of the art and future challenges of zeolites as catalysts. J. Catal. 216, 298–312. doi: 10.1016/S0021-9517(02)00132-X
Cundy, C. S., and Cox, P. A. (2003). The hydrothermal synthesis of zeolites: history and development from the earliest days to the present time. Chem. Rev. 103, 663–702. doi: 10.1021/cr020060i
Das, J., Satyanaryana, C. V. V., Chakrabarty, D. K., Piramanayagam, S. N., and Shringi, S. N. (1992). Substitution of Al in the AlPO4-5 and AlPO4-11 frameworks by Si and Fe: a study by Mössbauer, magic-angle-spinning nuclear magnetic resonance and electron paramagnetic resonance spectroscopies and chemical probes. J. Chem. Soc. Faraday Transact. 88, 3255–3261. doi: 10.1039/FT9928803255
Delgado, J. A., Águeda, V. I., Uguina, MA, Sotelo, J. L., and Fernández, P. (2013). Adsorption and diffusion of nitrogen, methane and carbon dioxide in aluminophosphate molecular sieve AlPO4-11. Adsorption 19, 407–422. doi: 10.1007/s10450-012-9463-6
Du, H., Fang, M., Xu, W., Meng, X., and Pang, W. (1997). Preparation by microwave irradiation of nanometre-sized AlPO4-5 molecular sieve. J. Mater. Chem. 7, 551–555. doi: 10.1039/a606132j
Feng, B., Li, J., Zhu, X., Guo, Q., Zhang, W., Wen, G., et al. (2016). The state of iron sites in the calcined FeAlPO4-5 and its tuning to the property of microporous AlPO4-5 molecular sieve. Catal. Today 263, 91–97. doi: 10.1016/j.cattod.2015.06.022
Gaber, S. A., Dawaymeh, F., Gaber, D. A., Basina, G., Ismail, I., Tharakshmy, A., et al. (2020). Combined DFT and experimental investigation on ion isomorphic substitution of aluminophosphate microporous crystals. Microp. Mesop. Mater. 294:109859. doi: 10.1016/j.micromeso.2019.109859
Guo, Z., Guo, C., Jin, Q., Li, B., and Ding, D. (2005). Synthesis and structure of large AlPO4-5 crystals. J. Porous Mater. 12, 29–33. doi: 10.1007/s10934-005-5230-3
Haszeldine, R. S. (2009). Carbon capture and storage: how green can black be? Science 325, 1647–1652. doi: 10.1126/science.1172246
Iwasaki, A., Sano, T., Kodaira, T., and Kiyozumi, Y. (2003). Growth behaviors of AFI type crystals. Microp. Mesop. Mater. 64, 145–153. doi: 10.1016/S1387-1811(03)00462-1
Kaneko, K., and Rodríguez-Reinoso, F. (2019). Nanoporous Materials for Gas Storage. New Jersey, NJ: Springer. doi: 10.1007/978-981-13-3504-4
Karanikolos, G. N., Garcia, H., Corma, A., and Tsapatsis, M. (2008). Growth of AlPO4–5 and CoAPO-5 films from amorphous seeds. Microp. Mesop. Mater. 115, 11–22. doi: 10.1016/j.micromeso.2007.11.051
Kontos, A. G., Likodimos, V., Veziri, C. M., Kouvelos, E., Moustakas, N., Karanikolos, G. N., et al. (2014). CO2 captured in zeolitic imidazolate frameworks: Raman spectroscopic analysis of uptake and host-guest interactions. ChemSusChem 7, 1696–1702. doi: 10.1002/cssc.201301323
Kueh, B., Kapsi, M., Veziri, C. M., Athanasekou, C., Pilatos, G., Reddy, K. S. K., et al. (2018). Asphaltene-derived activated carbon and carbon nanotube membranes for CO2 separation. Energy Fuels 32, 11718–11730. doi: 10.1021/acs.energyfuels.8b02913
Labropoulos, A., Veziri, C., Kapsi, M., Pilatos, G., Likodimos, V., Tsapatsis, M., et al. (2015). Carbon nanotube selective membranes with subnanometer, vertically aligned pores, and enhanced gas transport properties. Chem. Mater. 27, 8198–8210. doi: 10.1021/acs.chemmater.5b01946
Liu, Q., Cheung, N. C., Garcia-Bennett, A. E., and Hedin, N. (2011). Aluminophosphates for CO(2) separation. ChemSusChem 4, 91–97. doi: 10.1002/cssc.201000256
Liu, Q., Shi, J., Zheng, S., Tao, M., He, Y., and Shi, Y. (2014). Kinetics studies of CO2 adsorption/desorption on amine-functionalized multiwalled carbon nanotubes. Indus. Eng. Chem. Res. 53, 11677–11683. doi: 10.1021/ie502009n
Liu, Y., and Shen, L. (2008). From langmuir kinetics to first- and second-order rate equations for adsorption. Langmuir 24, 11625–11630. doi: 10.1021/la801839b
Loganathan, S., Tikmani, M., Edubilli, S., Mishra, A., and Ghoshal, A. K. (2014). CO2 adsorption kinetics on mesoporous silica under wide range of pressure and temperature. Chem. Eng. J. 256, 1–8. doi: 10.1016/j.cej.2014.06.091
Metz, B., Davidson, O., De Coninck, H., Loos, M., and Meyer, L. (2005). IPCC Special Report on Carbon Dioxide Capture and Storage, in, Intergovernmental Panel on Climate Change. Geneva: Working Group III.
Mohapatra, S. K., Sahoo, B., Keune, W., and Selvam, P. (2002). Synthesis, characterization and catalytic properties of trivalent iron substituted hexagonal mesoporous aluminophosphates. Chem. Commun. 14, 1466–1467. doi: 10.1039/b204215k
Musyoka, N. M., Petrik, L. F., Hums, E., Kuhnt, A., and Schwieger, W. (2015). Thermal stability studies of zeolites A and X synthesized from South African coal fly ash. Res. Chem. Intermed. 41, 575–582. doi: 10.1007/s11164-013-1211-3
Peri, J. B. (1971). Surface chemistry of AlPO4—a mixed oxide of Al and P. Discuss. Faraday Soc. 52, 55–65. doi: 10.1039/DF9715200055
Pilatos, G., Vermisoglou, E. C., Romanos, G. E., Karanikolos, G. N., Boukos, N., Likodimos, V., et al. (2010). A closer look inside nanotubes: Pore structure evaluation of anodized alumina templated carbon nanotube membranes through adsorption and permeability studies. Adv. Funct. Mater. 20, 2500–2510. doi: 10.1002/adfm.200901429
Pokhrel, J., Bhoria, N., Wu, C., Reddy, K. S. K., Margetis, H., Anastasiou, S., et al. (2018). Cu- and Zr-based metal organic frameworks and their composites with graphene oxide for capture of acid gases at ambient temperature. J Solid State Chem. 266, 233–243. doi: 10.1016/j.jssc.2018.07.022
Rajic, N., and Kaucic, V. (2002). Molecular Sieves: Aluminophosphates, in: Encyclopedia of Catalysis. Singapore: John Wiley & Sons, Inc., doi: 10.1002/0471227617.eoc151
Resnik, K. P., Yeh, J. T., and Pennline, H. W. (2004). Aqua ammonia process for simultaneous removal of CO2, SO2 and NOx. Int. J. Environ. Technol. Manage. 4, 89–104. doi: 10.1504/IJETM.2004.004634
Sawant, S. Y., Munusamy, K., Somani, R. S., John, M., Newalkar, B. L., and Bajaj, H. C. (2017). Precursor suitability and pilot scale production of super activated carbon for greenhouse gas adsorption and fuel gas storage. Chem. Eng. J. 315, 415–425. doi: 10.1016/j.cej.2017.01.037
Sayari, A., Belmabkhout, Y., and Serna-Guerrero, R. (2011). Flue gas treatment via CO2 adsorption. Chem. Eng. J. 171, 760–774. doi: 10.1016/j.cej.2011.02.007
Schlegel, M. C., Grzimek, V., Guenther, G., Svetogorov, R., Veziri, C. M., Kapsi, M., et al. (2018). Explaining water adsorption in one-dimensional channels in AlPO4-5 on molecular scale. Microp. Mesop. Mater. 304:109201. doi: 10.1016/j.micromeso.2018.11.025
Serna-Guerrero, R., and Sayari, A. (2010). Modeling adsorption of CO2 on amine-functionalized mesoporous silica. 2: kinetics and breakthrough curves. Chem. Eng. J. 161, 182–190. doi: 10.1016/j.cej.2010.04.042
Simmons, J. M., Wu, H., Zhou, W., and Yildirim, T. (2011). Carbon capture in metal–organic frameworks—a comparative study. Energy Environ. Sci. 4, 2177–2185. doi: 10.1039/c0ee00700e
Sircar, S., and Golden, T. C. (1995). Isothermal and isobaric desorption of carbon dioxide by purge. Indus. Eng. Chem. Res. 34, 2881–2888. doi: 10.1021/ie00047a042
Siriwardane, R. V., Shen, M.-S., Fisher, E. P., and Poston, J. A. (2001). Adsorption of CO2 on molecular sieves and activated carbon. Energy Fuels 15, 279–284. doi: 10.1021/ef000241s
Stoeger, J. A., Veziri, C. M., Palomino, M., Corma, A., Kanellopoulos, N. K., Tsapatsis, M., et al. (2012). On stability and performance of highly c-oriented columnar AlPO <inf>4 </inf>-5 and CoAPO-5 membranes. Microp. Mesop. Mater. 147, 286–294. doi: 10.1016/j.micromeso.2011.06.028
Tabish, A., Varghese, A. M., Wahab, M. A., and Karanikolos, G. N. (2020). Perovskites in the energy grid and CO2 conversion: current context and future directions. Catalysts 10:95. doi: 10.3390/catal10010095
Tang, Z. K., Sun, H. D., Wang, J., Chen, J., and Li, G. (1998). Mono-sized single-wall carbon nanotubes formed in channels of AlPO4-5 single crystal. Appl. Phys. Lett. 73, 2287–2289. doi: 10.1063/1.121704
Utchariyajit, K., and Wongkasemjit, S. (2008). Structural aspects of mesoporous AlPO4-5 (AFI) zeotype using microwave radiation and alumatrane precursor. Microp. Mesop. Mater. 114, 175–184. doi: 10.1016/j.micromeso.2008.01.002
Varghese, A. M., and Karanikolos, G. N. (2020). CO2 capture adsorbents functionalized by amine – bearing polymers: A review. Int. J. Greenhouse Gas Control 96:103005. doi: 10.1016/j.ijggc.2020.103005
Wan, Y., Williams, C. D., Duke, C. V. A., and Cox, J. J. (2000). Systematic studies on the effect of water content on the synthesis, crystallisation, conversion and morphology of AlPO4-5 molecular sieve. J. Mater. Chem. 10, 2857–2862. doi: 10.1039/b006127l
Wei, W., Moulijn, J. A., and Mul, G. (2008). Effect of steaming of iron containing AlPO-5 on the structure and activity in N2O decomposition. Microp. Mesop. Mater. 112, 193–201. doi: 10.1016/j.micromeso.2007.09.030
Wilson, S. T., Lok, B. M., Messina, C. A., Cannan, T. R., and Flanigen, E. M. (1982). Aluminophosphate molecular sieves: a new class of microporous crystalline inorganic solids. J. Am. Chem. Soc. 104, 1146–1147. doi: 10.1021/ja00368a062
Yazaydin, A. Ö., Snurr, R. Q., Park, T.-H., Koh, K., Liu, J., LeVan, M. D., et al. (2009). Screening of metal–organic frameworks for carbon dioxide capture from flue gas using a combined experimental and modeling approach. J. Am. Chem. Soc. 131, 18198–18199. doi: 10.1021/ja9057234
Yu, Y., Li, X., Krishna, R., Liu, Y., Cui, Y., Du, J., et al. (2018). Enhancing CO2 adsorption and separation properties of aluminophosphate zeolites by isomorphous heteroatom substitutions. ACS Appl. Mater. Interfaces. 10, 43570–43577. doi: 10.1021/acsami.8b11235
Zhang, S., Cui, M., Zheng, J., and Meng, C. (2020). Insights of the crystallization process of AlPO4-5 using hexamethyleneimine as template. Solid State Sci. 105:106291. doi: 10.1016/j.solidstatesciences.2020.106291
Zhang, Z., Zhang, W., Chen, X., Xia, Q., and Li, Z. (2010). Adsorption of CO2 on zeolite 13X and activated carbon with higher surface area. Separat. Sci. Technol. 45, 710–719. doi: 10.1080/01496390903571192
Keywords: aluminophosphates, adsorption, carbon dioxide, AFI, metal substitution, zeolites, adsorption kinetics, PSA
Citation: Papageorgiou A, Reddy KSK, Karonis D, Reinalda D, Al Wahedi Y and Karanikolos GN (2020) Morphology, Activation, and Metal Substitution Effects of AlPO4-5 for CO2 Pressure Swing Adsorption. Front. Chem. 8:568669. doi: 10.3389/fchem.2020.568669
Received: 01 June 2020; Accepted: 24 August 2020;
Published: 06 October 2020.
Edited by:
Svetlana Ivanova, University of Seville, SpainReviewed by:
Cristina Megías-Sayago, Université de Strasbourg, FranceLaura Pastor-Pérez, University of Surrey, United Kingdom
Copyright © 2020 Papageorgiou, Reddy, Karonis, Reinalda, Al Wahedi and Karanikolos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Georgios N. Karanikolos, georgios.karanikolos@ku.ac.ae
 Andreas Papageorgiou
Andreas Papageorgiou K. Suresh Kumar Reddy
K. Suresh Kumar Reddy Dimitrios Karonis
Dimitrios Karonis Donald Reinalda
Donald Reinalda Yasser Al Wahedi1,3
Yasser Al Wahedi1,3  Georgios N. Karanikolos
Georgios N. Karanikolos