Dysregulated Plasma Membrane Turnover Underlying Dendritic Pathology in Neurodegenerative Diseases
- Department of Brain and Cognitive Sciences, Daegu Gyeongbuk Institute of Science and Technology (DGIST), Daegu, South Korea
Due to their enormous surface area compared to other cell types, neurons face unique challenges in properly handling supply and retrieval of the plasma membrane (PM)—a process termed PM turnover—in their distal areas. Because of the length and extensiveness of dendritic branches in neurons, the transport of materials needed for PM turnover from soma to distal dendrites will be inefficient and quite burdensome for somatic organelles. To meet local demands, PM turnover in dendrites most likely requires local cellular machinery, such as dendritic endocytic and secretory systems, dysregulation of which may result in dendritic pathology observed in various neurodegenerative diseases (NDs). Supporting this notion, a growing body of literature provides evidence to suggest the pathogenic contribution of dysregulated PM turnover to dendritic pathology in certain NDs. In this article, we present our perspective view that impaired dendritic endocytic and secretory systems may contribute to dendritic pathology by encumbering PM turnover in NDs.
Introduction
Dendrites are neuronal compartments essential for receiving electrochemical signals from presynaptic neurons through formed synapses. Accurate neuronal wiring relies critically on the proper establishment of the dendritic field that is achieved by both structural build-ups of dendritic arbors and functional maturation of synapses (Jan and Jan, 2010). The establishment of the dendritic field is by nature a dynamic process as it is inevitably accompanied by dramatic changes in the morphology of entire dendritic arbors. Even after the establishment of the dendritic field, neuronal connections can be rewired in response to changes in the external environment by dynamically altering dendritic morphology and readjusting formed synapses. Therefore, disruption of dendritic morphology will invariably lead to failed synapse formation and communication between neurons.
To maintain dendritic morphology and dynamics, neurons need a constant turnover of plasma membranes (PMs). This process of PM turnover is mediated primarily by endocytic and secretory pathways. However, due to its highly elaborate dendrites, a typical neuron has a 10,000 times larger surface area than does a typical epithelial cell (Horton and Ehlers, 2004). Thus, a neuron will undoubtedly face a staggering challenge to grow and maintain those dendrites if it were to rely solely on somatic endocytic and secretory systems (Pfenninger, 2009). Thankfully, neuronal dendrites showcase various types of endocytic and secretory components, which participate in dendritic growth and maintenance, as well as a local supply of PM proteins (Jan and Jan, 2010; Puram and Bonni, 2013; Kennedy and Hanus, 2019).
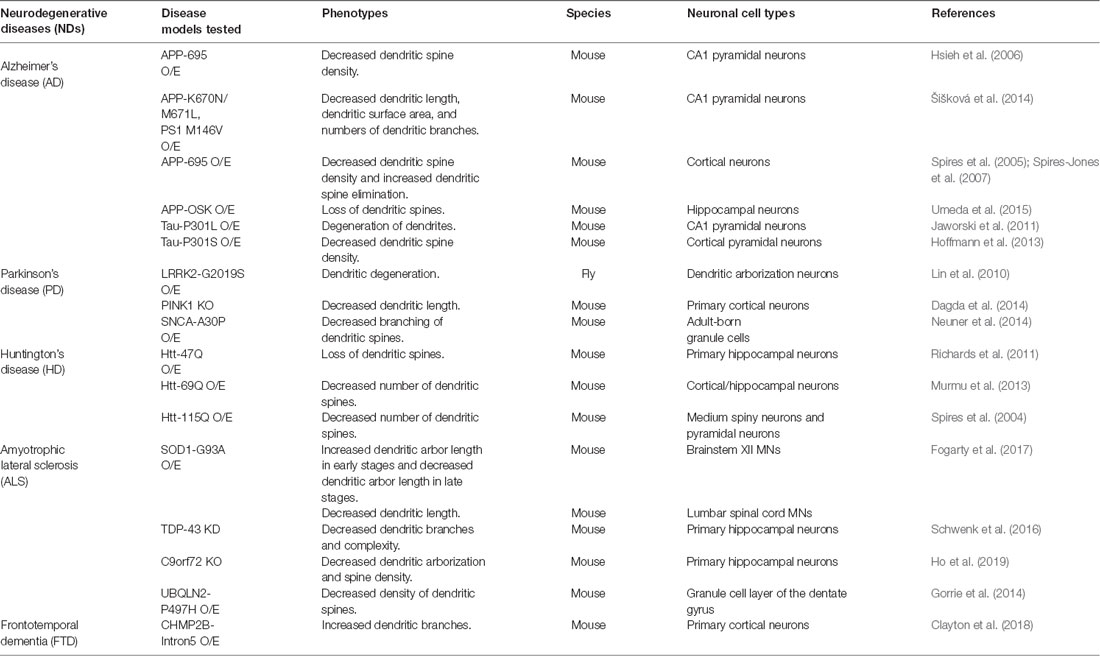
Dendritic changes are frequently observed in animal models of various neurodegenerative diseases (NDs), such as Alzheimer’s disease (AD), Parkinson’s disease (PD), polyglutamine (polyQ) diseases, and amyotrophic lateral sclerosis (ALS; summarized in Table 1). Consistently, dendritic pathology has been reported in post-mortem brain samples of patients with these diseases (Mehraein et al., 1975; Graveland et al., 1985; Nakano and Hirano, 1987; Patt et al., 1991; Ferrer, 1999; Kulkarni and Firestein, 2012). Although affected neuronal cell types and the patterns of dendritic changes vary depending on the disease, NDs generally share common pathological features such as decreased dendritic complexity and impaired synaptic maturation (Kulkarni and Firestein, 2012; Herms and Dorostkar, 2016). Previous studies identified several molecules and cellular processes involved in dendritic pathology in NDs. For example, a recent study identified a transcription factor Forkhead Box O (FOXO) whose sequestration by nucleus-accumulated toxic polyQ proteins in Drosophila sensory neurons results in dendritic defects (Kwon et al., 2018). In AD, β-amyloid (Aβ) has been reported to cause dendritic spine loss and to decrease expression of AMPA receptor on the synaptic surface by enhancing endocytosis in CA1 pyramidal neurons (Hsieh et al., 2006). In a PD model, knockout of Pink1 showed a shortening of dendritic lengths presumably through disrupting mitochondrial transport in mouse primary cortical and midbrain neurons (Dagda et al., 2014). In a UBQLN2-P497H mouse model of ALS, impairment of the protein degradation system led to dendritic spinopathy accompanied by synaptic dysfunction, and cognitive deficits (Gorrie et al., 2014). Besides what we have described so far, many other molecules have been identified whose dysregulation interferes with cellular components such as cytoskeletons, mitochondria, endosomes, ER, and Golgi that may be linked to dendritic pathology (Jan and Jan, 2010; Lei et al., 2016; Kweon et al., 2017; Kelliher et al., 2019). Currently, how these cellular components contribute to dendritic pathology is being worked out in many labs. Here, we propose that dendritic endocytic and secretory pathways, when disrupted, may contribute to dendritic pathology in several NDs.
In this review article, we will first describe the general mechanisms of PM turnover mediated by endocytosis and exocytosis. Next, we will provide an overview of dendritic endocytic and secretory pathways. Afterward, we will discuss how local molecular machinery might regulate dendritic endocytic and secretory pathways for PM turnover and how their dysfunction might contribute to dendritic pathology in several NDs. Finally, we will propose how dendritic endocytic and secretory pathways might be linked to selective dendritic vulnerability in NDs.
Basic Mechanisms of PM Turnover in Neurons: Endocytosis and Exocytosis
PM turnover is defined as the process by which membranes are continuously cycled to and from the PM. Through this process a cell can: (1) expand or reduce its size; (2) alter its shape; and (3) insert or remove from its PM the membranous lipids and proteins needed to convey both intra- and extra-cellular signals.
How is PM turnover regulated in neurons? Exocytosis and endocytosis are thought to be the primary means by which expansion and retrieval of the PM are mediated, respectively (Pfenninger, 2009; Peng et al., 2015). In a typical cell, materials that comprise the PM are first synthesized in the endoplasmic reticulum (ER) and then are modified and sorted in Golgi, from where vesicles bud and are inserted into the PM by exocytosis. In yeast, exocytosis of these PM-expanding vesicles requires tethering to PM by exocyst, without which soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complexes required for the membrane fusion do not form (TerBush et al., 1996; Grote et al., 2000). In neurons, their contribution to the growth of neurites (Vega and Hsu, 2001; Murthy et al., 2003)—and more specifically dendrites (Peng et al., 2015; Zou et al., 2015; Lira et al., 2019)—has been observed in Drosophila and cultured mammalian neurons. Interestingly, exocyst seems to be dispensable for neurotransmitter secretion in Drosophila (Murthy et al., 2003; Mehta et al., 2005), but not in primary hippocampal neurons (Lira et al., 2019). Generally, for membranes to fuse, SNARE proteins must be present on both membranous systems (Südhof and Rothman, 2009). For instance, Urbina et al. (2018) showed that VAMP2-positive exocytic vesicles contribute to PM expansion in neurites of mouse cortical neurons. Another SNARE protein, tetanus neurotoxin-insensitive (TI)-VAMP, has been shown to contribute to both axonal and dendritic growth without affecting synaptic vesicle fusion in primary neuronal cultures (Coco et al., 1999; Martinez-Arca et al., 2000, 2001). However, a knockout of TI-VAMP in mice only partially limited neurite outgrowth, suggesting that other SNARE proteins may mediate PM expansion (Meldolesi, 2011; Sato et al., 2011). In 2014, another group showed that the exocytosis of VAMP4-positive vesicles seems to contribute to the neurite growth in PC12 cells (Colombo et al., 2014). Interestingly, another SNARE protein, Sec22b, has been shown to contribute to PM expansion in neurons probably by mediating lipid transfer from ER to PM without vesicular fusion (Petkovic et al., 2014).
Endocytosis is the primary means by which PM is internalized, which may offset the functions of exocytosis. In Drosophila C4 dendritic arborization (da) neurons, defects in exocytosis-mediated dendritic growth were mitigated by blocking clathrin-mediated endocytosis (CME) using a temperature-sensitive dominant-negative allele of shibire (shits1, Peng et al., 2015). Urbina et al. (2018) also showed that CME contributes to the retrieval of PM in shaping neurite growth in mouse cortical neurons and suggested that exocytosis-mediated PM expansion in neurites can be counterbalanced by CME. Some of these endocytic vesicles, once internalized via endocytosis, may directly fuse with medial/trans-Golgi, at least in yeast (Day et al., 2018). In general, however, most other endocytic vesicles fuse with early endosomes (EEs), wherein sorting of the PM components takes place. Those components may be rapidly recycled back to the PM from EEs or slowly via recycling endosomes (REs; Taguchi, 2013). As EEs mature into late endosomes (LEs) en route to degradation (Huotari and Helenius, 2011), some of the PM components are recycled back to Golgi via retromers (Chen et al., 2019). Collectively, these two processes—endocytosis and exocytosis—regulate PM turnover in a typical cell, likely including neurons (Figure 1).
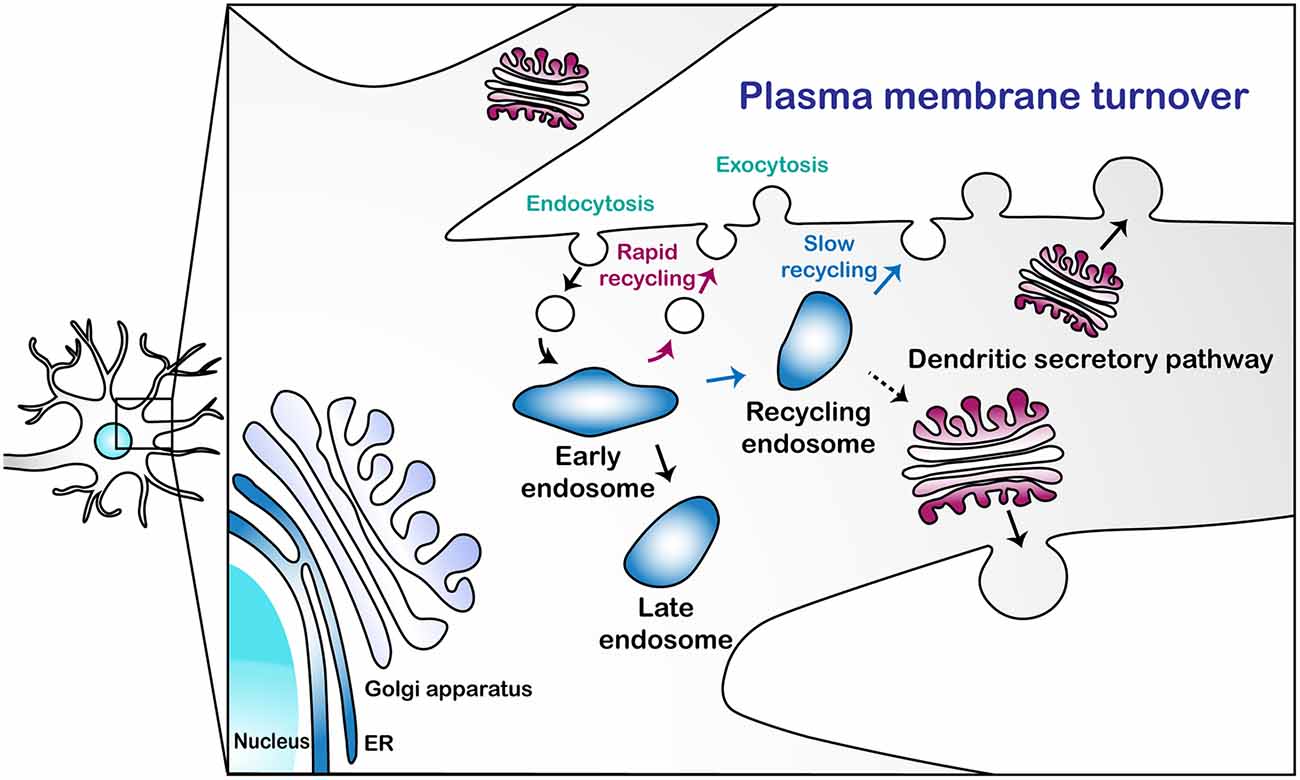
Figure 1. A schematic illustration of the plasma membrane turnover in neuronal dendrites. This illustration describes three pathways for plasma membrane (PM) turnover: rapid endosomal recycling via early endosomes (EEs); slow endosomal recycling via recycling endosomes (REs); and secretion via the dendritic secretory pathway.
The Presence of Dendritic Endocytic and Secretory Pathways and Their Potential Link to Dendritic Morphology in Neurons
Although it is fairly well established that the endocytic and secretory pathways, in general, regulate dendritic morphology via PM turnover, the extent to which dendritic endocytic and secretory pathways partake in regulating dendritic morphology via local PM turnover is less clear. Although the endocytic organelles have been fairly well delineated in dendrites, secretory organelles in dendrites have remained more elusive. Here, we will briefly discuss several dendritic endocytic and secretory units and their potential relevance to dendritic morphology.
Dendritic Endocytic Pathway
All the major types of endosomes—EEs, LEs, and REs—have been shown to exist in dendrites. Endocytosis in dendrites has been reported to play a major role in Drosophila dendritic pruning by triggering dendritic thinning via internalizing PM (Kanamori et al., 2015) and through internalizing specific cell adhesion molecule, Neuroglian (Zhang et al., 2014). Although endocytosis is one of the means by which EEs are produced (Mellman, 1996), blocking endosomal transport from soma to dendrites leads to depletion of EEs in dendrites of Drosophila da neurons (Satoh et al., 2008; Zheng et al., 2008), suggesting that most of the EEs in dendrites may be derived from the soma. Intriguingly, a recent study shows that the trans-Golgi network (TGN), but not endocytosis, is indispensable in forming Rab5-labeled EEs in yeast (Nagano et al., 2019). However, whether EEs can be synthesized from TGN in neurons, let alone neuronal dendrites, has not been shown.
LEs and REs, which are thought to be derived from EEs (Mellman, 1996), are also detected in dendrites and/or dendritic spines (Cooney et al., 2002; Cheng et al., 2018; Yap et al., 2018). LEs are well known for their role in sorting ubiquitinated proteins for degradation via lysosomes (Hu et al., 2015). In dendrites, they transport dendritic cargos towards the soma for degradation via lysosomes (Cheng et al., 2018; Yap et al., 2018). However, one study showed that LEs translocate to and fuse with the PM after making repeated contact with the ER. This process was shown to contribute to neurite growth in PC12 cells (Raiborg et al., 2015). REs have been shown to mediate PM turnover process in dendrites and dendritic spines, thereby mediating their growth in rat hippocampal or cortical neurons (Park et al., 2006; Bowen et al., 2017). Interestingly, REs have been shown to exchange cargoes and make physical contact with Golgi in Drosophila, sea urchin embryos, and mammals (Mallard et al., 1998; Fujii et al., 2020a,b). However, their interaction in neuronal dendrites has not been reported. Further examining the potential interplay between these different types of endosomes and secretory organelles, such as ER and Golgi, in dendrites may provide significant insight on the mechanisms underlying PM turnover.
Dendritic Secretory Pathway
The ER that localizes in the dendritic spine, called spine apparatus (SA), has a specialized membrane-stacked morphology similar to that of Golgi (Gray, 1959a,b). Based on its morphology, SA was speculated to play a role in dendritic secretory function, though it did not garner any significant experimental support for a long time. In 2001, through post-embedding immunogold labeling in adult rat tissue, Pierce et al. (2001) showed in neuronal dendrites the presence of a repertoire of proteins that localize to ER, ER-Golgi intermediate compartment (ERGIC), and Golgi, indicative of a presence of dendritic secretory pathway. Interestingly, although some of those proteins were found nearby SA in dendritic spines, others were found within dendrites away from SA. In contrast, a couple of subsequent studies were not able to verify this finding in cultured neurons (Hanus et al., 2014; Bowen et al., 2017). Whether or not the difference in sample type is accountable for this apparent discrepancy remains to be tested.
Another distinct secretory organelle found in dendrites is termed Golgi outpost (GOP), which was first defined in cultured hippocampal neurons (Horton and Ehlers, 2003). A correlation between the localization of GOPs at branch points and dendritic growth in both Drosophila (Ye et al., 2007; Lin et al., 2015) and mammals (Horton et al., 2005; Ye et al., 2007) supports the purported function of GOPs in dendritic growth via PM supply. In the branch points, GOPs have been shown to function in supplying PM proteins, such as BDNF (Horton and Ehlers, 2003), ADAM10 (Saraceno et al., 2014), Kainate receptors (Evans et al., 2017), and NMDA receptors (Jeyifous et al., 2009) in mammalian hippocampal neurons. The potential role of GOPs in the dendritic pathology of neurological disorders has been recently discussed in a review article (Caracci et al., 2019). However, GOP seems to be relatively rare in mammalian neurons (Horton et al., 2005; Hanus et al., 2014; Bowen et al., 2017), and even absent in mouse Purkinje cells 2 weeks after birth (Liu et al., 2017). Therefore, the importance of GOPs in dendritic morphology in adult neurons remains to be further elucidated.
Relatively recently characterized dendritic secretory units further complicate our understanding of the dendritic secretory pathway. ERGIC is normally sandwiched in between ER and cis-Golgi but is scattered all over the dendrites of rat hippocampal (Hanus et al., 2014) or cortical neurons (Bowen et al., 2017). In dendrites, ERGIC seems to perform a secretory function, bypassing Golgi entirely (Hanus et al., 2014; Bowen et al., 2017). However, by using a highly specific Golgi marker, pGolt, another study provides evidence that a small (200–1,000 nm in diameter) Golgi membrane compartment, termed Golgi satellite (GS), exists in between ERGIC and retromer in dendrites and participates in local PM turnover in rat hippocampal primary neurons (Mikhaylova et al., 2016). The authors further show that GSs are more numerous than GOPs and are positive for some Golgi markers, but not for all. Considering that cis-, medial-, and trans-Golgi compartments often exist separately in Drosophila da neuronal dendrites (Zhou et al., 2014), failure to detect Golgi compartments in dendrites of other types of neurons by some other groups may be due to the simplified structure of dendritic Golgi, which may be missing some structural proteins that are often used to label Golgi, such as GM130 (Zhou et al., 2014). Overall, the dendritic secretory pathway is extremely complicated and there is still much left to be discovered.
The Local Molecular Machinery That May Regulate Dendritic Endocytic and Secretory Pathways for PM Turnover
Rab GTPases as Potent Local Regulators of the Endocytic Pathway
Ras-related in brain (Rab) GTPase proteins are among the most compelling candidate molecular machinery that may play crucial roles in (dendritic) PM turnover. Rabs, which were first found in rat brains (Salminen and Novick, 1987), are significantly conserved among eukaryotes from yeast to human (Rojas et al., 2012). To date, more than 60 different Rabs have been identified in humans (Kiral et al., 2018). Rabs are the largest group of proteins in the Ras superfamily and function as molecular switches in diverse cellular contexts (Zhen and Stenmark, 2015); they are master regulators of membrane transport between organelles, or between an organelle and PM (Wandinger-Ness and Zerial, 2014). Given these known generalized functions, Rabs will likely play a major role in neuronal dendritic PM turnover.
Regulation of Rab Activity by Switching Its Guanine Nucleotide Status
The activity of Rab is determined by its guanine nucleotide status: GTP-bound is active and GDP-bound is inactive. Regulatory proteins, such as Rab guanine nucleotide exchange factors (Rab GEFs), Rab GTPase-activating proteins (Rab GAPs), and Rab-GDP dissociation inhibitors (Rab GDIs) control the guanine nucleotide status of Rab (Welz et al., 2014). Rab GEFs facilitate the release of GDP from Rabs, which allows them to bind GTP (Stenmark, 2009). GTP-bound active Rabs are then targeted to the particular membrane site where they collect effector proteins, such as sorting adaptors, tethering factors, kinases, phosphatases, and motor proteins, through which vesicle trafficking is mediated between membranous compartments. On the other hand, Rab GAPs catalyze the hydrolysis of GTP into GDP. Subsequently, Rab GDI binds to Rab-GDP, extracts it away from the membrane, and stabilizes this inactive form of Rab in the cytosol by preventing it from releasing GDP.
Characterized Roles of Rab GTPases in PM Turnover via the Endocytic Pathway
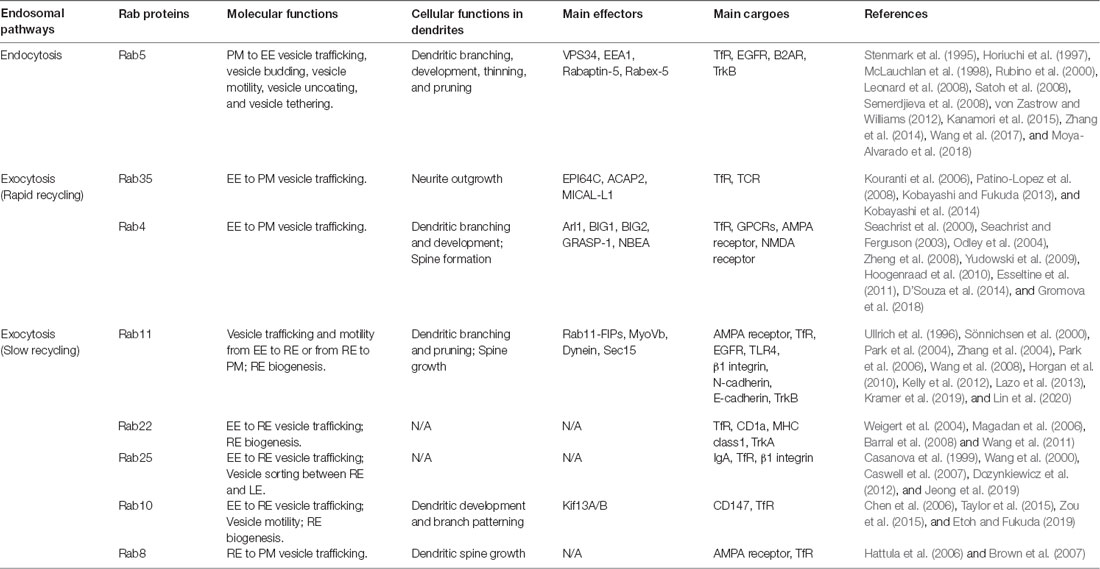
Endosomal membrane trafficking can be broadly divided into two different pathways: PM internalization to endosomal compartments and recycling vesicles from endosomes to PM. In this review article, we briefly explain the roles of various Rabs associated with each pathway (summarized in Table 2).
PM to EE
Retrieval of the PM is mediated by the internalization of a portion of PM, mostly via CME (Bitsikas et al., 2014). The endocytic vesicles are then targeted to EEs by Rab5. PM proteins, such as Transferrin Receptor (TfR), epidermal growth factor receptor (EGFR; Leonard et al., 2008), and β-2-adrenergic receptor (β2AR; von Zastrow and Williams, 2012), are reported to be transported to EEs via this endocytic pathway.
How Rab5 regulates this endocytic pathway is relatively well known. First, Rab5-GDI and adaptor protein 2 (AP2) complexes initiate vesicle budding from PM at clathrin-coated pits (McLauchlan et al., 1998). Rab5-vesicles then uncoat AP2 adaptor complexes and coat proteins, a process required for vesicle fusion with EEs (Semerdjieva et al., 2008). Lastly, Rab5 recruits various effectors, such as VPS34, EEA1, and Rabaptin-5/Rabex-5 complex (Stenmark et al., 1995; Horiuchi et al., 1997; Rubino et al., 2000), through which the endocytic vesicles dock and fuse with EE membrane.
Endosomes to PM
The PM is recycled mostly through two distinct endosomal pathways: the rapid recycling (1–5 min) pathway, through which membrane vesicles are transported directly from EEs to PM; and the slow recycling (10–20 min) pathway, through which membrane vesicles are transported to PM via REs (Jonker et al., 2020).
Rapid Recycling
TfR is among the well-characterized membrane proteins that go through the rapid recycling pathway. This pathway is known to be selectively blocked by knockdown or knockout of Rab35 (Kouranti et al., 2006), or by overexpression of its dominant-negative form (Patino-Lopez et al., 2008). In addition to Rab35, Rab4 also plays a crucial role in the regulation of TfR recycling via the rapid recycling pathway. Rab4 is primarily located around the exit sites of EE (EEES), where membrane fission actively occurs (Stenmark, 2009). At EEES, Rab4 recruits effectors, thereby promoting the Class I ARF cascade. It has been shown that inhibition of Rab4 effectors disrupts the elongated tubular formation of EE, an important process in the rapid recycling pathway (D’Souza et al., 2014). Consistently, when Rab4 was inhibited by overexpressing its dominant-negative form in HEK293 cells, TfR rapid recycling was perturbed (Yudowski et al., 2009).
Slow Recycling
RE is defined as a membranous compartment positive for Rab11 (Grant and Donaldson, 2009). Fluorescent live imaging shows that RE is generated by tubule elongation of EE, from which Rab5 gradually disappears and is replaced by Rab11 (Sönnichsen et al., 2000). Rab11 works together with numerous other Rabs and their effectors to engage in the overall process of slow recycling of various membrane proteins, such as AMPA receptor, rhodopsin, EGFR, TLR4, β1 integrin, N-cadherin, and E-cadherin (Kelly et al., 2012). In the following paragraphs, we will describe the slow recycling pathway, which comprises two continuous processes: EE-to-RE vesicle trafficking and RE-to-PM vesicle targeting.
In EE-to-RE trafficking, Rab10, Rab11, Rab22, and Rab25 are reported to be associated with this process as depletion or expression of dominant-negative forms of these proteins showed a decreased number of REs or inhibited RE biogenesis in diverse cell types (Wang et al., 2000; Weigert et al., 2004; Chen et al., 2006; Barral et al., 2008). For example, it seems that Rab11 and its effectors, such as Rab11 family interacting proteins (Rab11-FIPs) and microtubule motor proteins, are associated with EE-to-RE trafficking (Welz et al., 2014). Specifically, the Rab11 family interacting protein3 (Rab11-FIP3) complex was shown to directly interact with dynein light intermediate chain 1 (DLIC-1) and disruption of FIP3 binding with DLIC-1 inhibited EE-to-RE trafficking of TfR in epidermal carcinoma human cells (Horgan et al., 2010).
Vesicle targeting from REs to PM is achieved by the cooperation of Rab8 and Rab11. According to a previous study in hippocampal CA1 neurons, Rab11 translocates AMPA receptor-containing vesicles from the dendritic shaft to the dendritic spine. Then, Rab8 directly drives the insertion of AMPA receptor-containing vesicles into the synaptic membrane (Brown et al., 2007). This process is known to involve the actin cytoskeleton, which facilitates the movement of these vesicles. Myosin-Vb (MyoVb), an actin motor protein that can form a complex with Rab11 and FIP-2, directly mediates RE-to-PM vesicle transport (Wang et al., 2008). For tethering of vesicles coming from REs to PM, the interaction between Rab11 and the exocyst complex is required. One of the Rab11 effectors, Sec15, plays an important role in this process (Zhang et al., 2004). Once Sec15 binds to Rab11, they initiate sequential recruitment of exocyst complex subunits including cytoplasmic Exo84, Sec5, Sec6, Sec8, and Sec10, and PM-attached Exo70 and Sec3 (Zhang et al., 2004; Heider and Munson, 2012). They directly link the vesicle membrane and PM to promote targeted fusion of Rab11 vesicles with the PM.
Evidence for the Regulatory Roles of Rabs in Dendrite Morphogenesis
One of the best examples of experimental evidence for the involvement of early endosomal Rabs in dendrite morphogenesis comes from a study by Satoh et al. (2008), who showed in Drosophila class IV da (C4 da) neurons that mutation of a dynein subunit gene, dlic, led to proximally “bushy” dendrites and that dlic and Rab5 double mutation resulted in greatly simplified dendritic morphology. Interestingly, this double mutant phenotype was similar to those seen in neurons with Rab5 mutation only. These data indicate that Rab5, in a co-operation with dlic, plays a regulatory role in dendrite morphogenesis. Another study on the genetic interaction between Protein Kinase A (PKA) and Rab5 in C4 da neurons showed that PKA could also contribute to the dendritic arbor development by altering Rab5-endosomal transport in dendrites (Copf, 2014). More recently, it was reported that BDNF-induced dendritic branching accompanied increased number and mobility of TrkB-positive Rab5-endosomes in cultured rat hippocampal neurons (Moya-Alvarado et al., 2018). Accordingly, expression of the dominant-negative form of Rab5 reduced dendritic arborization which was partially rescued by BDNF treatment.
Many studies also described the association between Rab35 and Rab4 with dendrites. Rab35 was shown to recruit a series of effectors, such as MICAL-L1, ACAP2, and EHD1, to inactivate ARF6 (Kobayashi and Fukuda, 2013) and promote vesicle targeting from REs to neurite tips, thereby inducing neurite outgrowth in PC12 cells (Kobayashi et al., 2014). Rab4-positive endosomes have been associated with the dendritic formation in Drosophila C4 da neurons (Zheng et al., 2008). They showed that dlic mutants induced proximal shift in both Rab4-positive endosomes and dendritic branch distribution. However, dlic mutants also altered localization of GOPs, suggesting that the proximal shift in the branch distribution may be, at least in part, due to the mislocalization of both Rab4-positive endosomes and GOPs. Also, Rab4 is reported to collect its neuron-specific effector GRASP-1 to co-ordinate RE maturation, which is necessary for surface expression of AMPA receptor in dendrites of cultured rat hippocampal neurons (Hoogenraad et al., 2010). A more recent study showed that Rab4 forms a complex with GluN2B and VPS35 to regulate the surface expression and recycling of GluN2B-NMDA receptor in dendrites of cultured mouse hippocampal neurons (Gromova et al., 2018). In this process, active Rab4 collects Neurobeachin (NBEA), a Brain-enriched multi-domain protein, to link the complex with motor protein KIF21B, which enables vesicle trafficking. Deficiency of either NBEA or KIF21B results in decreased actin enrichment in dendritic spines and consequent reduction of dendritic spine number.
REs have been studied extensively in neuronal dendrites; Rab11 is the most prominent RE-associated molecule. The role of Rab11 in dendrites was initially highlighted by a collection of research from the same group (Park et al., 2004, 2006), who showed that LTP-inducing stimuli promoted the mobilization of Rab11-REs towards dendritic spines and vesicle fusion with PM, which resulted in rapid spine growth in hippocampal neurons. Moreover, expression of the dominant-negative form of Rab11 decreased total spine numbers, whereas overexpression of wild-type Rab11 increased them (Park et al., 2006). More recent studies have shown the involvement of Rab11-REs in dendritic pruning in Drosophila C4 da neurons (Kramer et al., 2019; Lin et al., 2020). These studies suggest that appropriate localization of Rab11-REs in dendrites is crucial for dendritic PM turnover and morphogenesis.
The function of Rab10 has also been associated with dendrite morphogenesis in C. elegans (Taylor et al., 2015; Zou et al., 2015). Taylor et al. (2015) reported that Rab10 mutants showed a reduction in posterior dendritic branches, but an increase in distal anterior branches in PVD neurons, indicating that Rab10 is a critical regulator of dendrite morphogenesis and patterning in C. elegans PVD sensory neurons.
Although these studies provide substantial evidence supporting the involvement of Rabs in dendrite morphogenesis, the mechanism by and the effectors with which they regulate dendritic PM turnover remain unclear. Further studies clarifying the exact regulatory roles of Rabs in dendrite morphogenesis would enrich our understanding of the physiological roles of the entire endosomal pathway in neurons.
Evidence for the Involvement of Local Rab-Mediated Endocytic Pathway in Dendritic Pathology in NDs
Several previous studies provide experimental evidence to support a link between endosomal defects and neuronal pathology in NDs, which has been well-reviewed in recent articles (Kiral et al., 2018; Guadagno and Progida, 2019). For example, in postmortem brains of AD patients, enlargement of Rab5-positive EEs and upregulation of Rab4 were observed in pyramidal neurons of the prefrontal cortex at the early-stages (Cataldo et al., 2000), and upregulation of Rab4, Rab5, Rab7, and Rab27 was observed in the cholinergic basal forebrain neurons (Ginsberg et al., 2011). In line with this, in animal models of HD, impaired conversion from Rab11-GDP to Rab11-GTP, and delayed TfR recycling back to PM were observed in primary cortical neurons (Li et al., 2009). Besides these general links between Rab-mediated endocytic pathway and dendritic pathology in NDs, more direct evidential links have been reported in studies using animal models of NDs. Umeda et al. (2015) reported that intracellular Aβ oligomers impaired endocytic vesicle trafficking of TfR in dendrites, which resulted in dendritic spine alteration in mouse primary neurons. Richards et al. (2011) showed that cultured hippocampal neurons expressing mutant huntingtin (htt) displayed a loss of dendritic spines when they were in proximity to htt aggregates and that this loss was due to functional defects in Rab11-mediated local endosomal recycling caused by the aggregates. Also, a previous study showed that the loss-of-function of TDP-43 in primary hippocampal neurons reduced the number and motility of Rab11-positive REs regulating NRG1-ErbB4-mediated trophic signaling in dendrites, thereby inducing dendritic defects (Schwenk et al., 2016). Another study showed that overexpression of mutant CHMP2B, which is associated with Frontotemporal dementia (FTD), in primary cortical neurons increased dendritic branches and decreased endolysosomal trafficking in dendrites (Clayton et al., 2018). Although the link between endosomal defects and dendritic pathology in a subset of NDs has been characterized as shown above, further studies on the details of underlying pathogenic mechanisms warrant further scrutiny.
COPI and COPII as Potential Local Regulators of the Secretory Pathway
No matter which dendritic secretory pathway is being considered, the early secretory pathway (from ER-to-Golgi or ER-to-ERGIC) seems to be involved. In this section, we will briefly outline the generalized characteristics of the early secretory pathway by describing some of its key regulators and make extensions to the dendritic secretory pathway and NDs where appropriate.
Regulation of COPII Vesicle Budding and Fusion in the Early Secretory Pathway
The secretory pathway comprises the transport of secretory and membranous materials from ER to Golgi and ultimately to PM. ER-to-ERGIC and ERGIC-to-Golgi in mammals and ER-to-Golgi transport in other less-developed species such as Drosophila and yeast are mediated by coat protein complex II (COPII) vesicles (Brandizzi and Barlowe, 2013). The COPII pathway is initiated from the ER exit site (ERES), a site on the ER that lacks ribosomes, which is defined by the presence of Sec16 (Hughes et al., 2009) anchored there by leucine-rich repeat kinase 2 (LRRK2; Cho et al., 2014). Sec16 recruits Sec12 (Montegna et al., 2012), a GEF for Sar1 (Barlowe and Schekman, 1993). Sar1, in turn, recruits the inner COPII components (Sec23–Sec24 complex; Matsuoka et al., 1998). Next, the outer COPII components (Sec13–31 complex) are recruited to and bind at the interface of the Sar1-Sec23 complex (Bi et al., 2007; Fromme et al., 2007). These inner and outer COPII components ultimately induce GTP hydrolysis of Sar1, which leads to the scission of COPII vesicles from the ER (Bielli et al., 2005; Fromme et al., 2007). Immediately after scission, the vesicles uncoat prior to fusion with the ERGIC or cis-Golgi (Suda et al., 2017). This fusion process is mediated by Rab1 GTPase on COPII vesicles and GM130 on the membranes of ERGIC or cis-Golgi (Sztul and Lupashin, 2009).
Regulation of COPI Vesicle Budding and Fusion in the Early Secretory Pathway
The transport process between the ER and Golgi is not unidirectional. The best characterized retrograde transport process from Golgi to ER is the COPI pathway (Spang, 2013; Arakel and Schwappach, 2018). COPI comprises γ-COP–δ-COP–ζ-COP–β-COP tetrameric complex and α-COP–β′-COP–ε-COP trimeric complex that forms inner and outer layers of the COPI coat, respectively (Eugster et al., 2000). These complexes are recruited to the Golgi membrane upon activation of the ADP-ribosylation factor (ARF). Once recruited to the Golgi membrane, the subunits α-COP, β′-COP, γ-COP, and δ-COP recognize specific motifs on cargoes and promote their incorporation into COPI vesicles (Cosson and Letourneur, 1994; Brandizzi and Barlowe, 2013). The scission of COPI vesicles is mediated by dimerization of ARF1 (Beck et al., 2008, 2011), and its GTP hydrolysis promotes the uncoating of COPI vesicles (Tanigawa et al., 1993) before fusing with the ER membrane via Dsl1 tethering complex in yeast (Andag and Schmitt, 2003; Ren et al., 2009) and likely the NAG-RINT1-ZW10 (NRZ) complex in mammals (Hirose et al., 2004; Civril et al., 2010). However, whether these processes are conserved in the dendritic secretory systems in neurons remains unclear.
Evidence for the Involvement of Dendritic Secretory Pathway in Dendritic Pathology
Although the origins of dendritic secretory units are mostly unknown, we suspect that they are not entirely discrete from the canonical secretory units in the soma. Indeed, a study reported that GOPs may originate from somatic Golgi in rat hippocampal neurons (Quassollo et al., 2015). Interestingly, functional and structural alterations of somatic Golgi, termed Golgi pathology (Gosavi et al., 2002; Liazoghli et al., 2005; van Dis et al., 2014), as well as impaired exocytosis mediated by the secretory pathway (Larsen et al., 2006; Spencer et al., 2016), has been frequently observed in neurons of animal models for NDs. Provided that the dendritic secretory system has some reliance on the canonical secretory system, these evidences suggest a possibility of widespread involvement of the dendritic secretory pathway in dendritic pathology.
A recent study on polyQ toxicity in Drosophila has also provided a link between the dendritic secretory pathway and dendritic pathology. Chung et al. (2017) showed that nucleus-accumulated polyQ proteins led to the reduction of the CrebA mRNA level. Because CrebA is the master regulator of the secretory pathway (Abrams and Andrew, 2005; Fox et al., 2010), polyQ toxicity led to the perturbation of the COPII pathway, thereby decreasing GOP formation, and ultimately resulting in reduced dendritic branches (Chung et al., 2017). Indeed, knockdown of Sec31 (Chung et al., 2017) or homozygotic mutation in Sar1 in Drosophila da neurons (Ye et al., 2007) reduced the number or integrity of GOPs, respectively. The disruption also led to a significantly decreased dendritic PM supply, although to what extent GOPs, rather than somatic Golgi, contribute to such decrease is difficult to tell. Interestingly, when GOPs were selectively ablated by laser, dendritic branch dynamics were reduced (Ye et al., 2007). However, the extent to which the laser-ablated GOPs were not measured nor did the authors examine other potential damage that may have been induced by the laser.
Glutamatergic excitotoxicity involving the NMDA receptor is often observed in animal models of NDs (Lewerenz and Maher, 2015). Interestingly, NMDA receptor trafficking in dendrites is mediated by dendritic ERES and GOPs (Aridor et al., 2004; Jeyifous et al., 2009). This evidences suggest that excitotoxicity involving NMDA receptors may be dependent on the dendritic secretory pathway. Upon knock-out of Lrrk2, Sec16A detached from the dendritic ERES, which led to the impairment of ER-to-Golgi transport and NMDA receptor trafficking in mouse primary hippocampal neurons (Cho et al., 2014). Also, overexpression of PD-linked LRRK2 mutants has been shown to induce NMDA receptor-mediated excitotoxicity, leading to dendritic degeneration in rat cortical neurons (Plowey et al., 2014). These evidences support a model that suggests that the dendritic secretory pathway is regulated by LRRK2 whose dysfunction in PD is associated with NMDA receptor-mediated excitotoxicity and dendritic degeneration. Interestingly, Lin et al. (2015) found that Lrrk, a Drosophila ortholog of LRRK2, co-localized with somatic Golgi and GOPs in Drosophila da neurons, and that overexpression of a PD-linked mutant form of LRRK2, LRRK2 G2019S, suppressed anterograde movements of GOPs marked by ManII-eGFP. This GOP transport defect may underlie the dendrite degeneration observed in LRRK2 G2019S-expressing Drosophila da neurons (Lin et al., 2010). Whether or not other dendritic secretory units are also linked to NDs awaits further investigation.
Conclusions and Perspectives
Neuronal dendrites seem to be highly vulnerable to neurotoxic insults, including those that arise in NDs (Luebke et al., 2010; Kulkarni and Firestein, 2012; Hasel et al., 2015; Kweon et al., 2017). This vulnerability may be partly due to differences between dendrites and soma in their response to stress, such as exposure to ROS or NMDA (Hasel et al., 2015). Here, we propose that dendritic endocytic and secretory pathways may be more susceptible than the canonical pathways to neurotoxicity, which could contribute to the vulnerability of dendrites in NDs.
Although the dendritic and the canonical pathways occur in distinct areas of the neuron, they share many of the regulatory molecules. Also, pieces of evidence show that at least parts of the dendritic secretory system, such as GOPs, may be derived from the canonical somatic secretory system (Quassollo et al., 2015), suggesting that the dendritic secretory system is under the purview of the canonical system in the soma. Thus, it is possible that when endocytic and secretory functions are under assault in neurons, the canonical system may need to limit its purview in dendrites to support its somatic functions. We posit several reasons in support of this possibility: (1) knockdown of Sec31 and nuclear polyQ expression lead to the loss of GOPs, but not somatic Golgi (Chung et al., 2017); (2) loss-of-function mutations of genes related to ER-to-Golgi trafficking, such as Sec31, Rab1, and Sar1, all lead to impaired arborization of dendrites, but normal morphology of axons in Drosophila da neurons (Ye et al., 2007); (3) a partial loss-of-function in Golgi SNARE protein Membrin causes neuron-specific dysfunctions and significantly impairs dendritic growth in a Drosophila model for progressive myoclonus epilepsy (Praschberger et al., 2017); (4) neurons often undergo dendritic degeneration before cell death in NDs (Klapstein et al., 2001; Jaworski et al., 2011; Fogarty et al., 2016); (5) shrinking dendritic area has been identified as an adaptive response to SCA1 toxicity (Dell’Orco et al., 2015); (6) dendrites in Drosophila motoneurons (Ryglewski et al., 2014) and da neurons (Shorey et al., 2020) have been shown to be dispensable for neuronal survival; and (7) endocytic and secretory dysfunctions are often observed in a number of NDs (Wang et al., 2020). These results may partly explain the fact that neuronal dendrites are more vulnerable to neurotoxicity than other neuronal domains (Luebke et al., 2010; Hasel et al., 2015; Kweon et al., 2017). Further investigations in the dendritic endocytic and secretory pathways will be needed to test the validity of our hypothesis in addressing the issue of dendritic vulnerability in NDs.
In this review article, we presented our perspective view that impaired PM turnover involving dysregulation of the dendritic endocytic and secretory pathways may contribute to dendritic pathology in NDs. Although there is a growing body of evidence for the potential link between impaired PM turnover and dendritic pathology in NDs, our understanding of the exact pathogenic mechanisms remains largely elusive. We propose that dendritic pathology in NDs may involve dysregulation of the regulatory machinery, such as Rab GTPases and COPI/COPII, for the dendritic endocytic and secretory pathways described above. Dysregulation of the dendritic pathways appears to complement cytoskeleton impairment as underlying pathogenic mechanisms for dendritic pathology. Because dendritic defects are often early features of ND, future studies to elucidate the pathogenic mechanisms by which impaired PM turnover contributes to dendritic pathology in NDs will deepen our understanding of the early pathogenesis of NDs.
Author Contributions
JP, CC, SP and SL conceptualized the theme of the review and wrote the manuscript together. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Science and Information and Communications Technology (ICT; 2018R1A2B6001607 and 2019R1A4A1024278) and the Development of Platform Technology for Innovative Medical Measurements Program from the Korea Research Institute of Standards and Science (KRISS-2019-GP2019-0018).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abrams, E. W., and Andrew, D. J. (2005). CrebA regulates secretory activity in the Drosophila salivary gland epidermis. Development 132, 2743–2758. doi: 10.1242/dev.01863
Andag, U., and Schmitt, H. D. (2003). Dsl1p, an essential component of the Golgi-endoplasmic reticulum retrieval system in yeast, uses the same sequence motif to interact with different subunits of the COPI vesicle coat. J. Biol. Chem. 278, 51722–51734. doi: 10.1074/jbc.M308740200
Arakel, E. C., and Schwappach, B. (2018). Formation of COPI-coated vesicles at a glance. J. Cell Sci. 131:jcs209890. doi: 10.1242/jcs.209890
Aridor, M., Guzik, A. K., Bielli, A., and Fish, K. N. (2004). Endoplasmic reticulum export site formation and function in dendrites. J. Neurosci. 24, 3770–3776. doi: 10.1523/JNEUROSCI.4775-03.2004
Barlowe, C., and Schekman, R. (1993). SEC12 encodes a guanine-nucleotide-exchange factor essential for transport vesicle budding from the ER. Nature 365, 347–349. doi: 10.1038/365347a0
Barral, D. C., Cavallari, M., McCormick, P. J., Garg, S., MaGee, A. I., Bonifacino, J. S., et al. (2008). CD1a and MHC class I follow a similar endocytic recycling pathway. Traffic 9, 1446–1457. doi: 10.1111/j.1600-0854.2008.00781.x
Beck, R., Prinz, S., Diestelkotter-Bachert, P., Rohling, S., Adolf, F., Hoehner, K., et al. (2011). Coatomer and dimeric ADP ribosylation factor 1 promote distinct steps in membrane scission. J. Cell Biol. 194, 765–777. doi: 10.1083/jcb.201011027
Beck, R., Sun, Z., Adolf, F., Rutz, C., Bassler, J., Wild, K., et al. (2008). Membrane curvature induced by Arf1-GTP is essential for vesicle formation. Proc. Natl. Acad. Sci. U S A 105, 11731–11736. doi: 10.1073/pnas.0805182105
Bi, X., Mancias, J. D., and Goldberg, J. (2007). Insights into COPII coat nucleation from the structure of Sec23.Sar1 complexed with the active fragment of Sec31. Dev. Cell 13, 635–645. doi: 10.1016/j.devcel.2007.10.006
Bielli, A., Haney, C. J., Gabreski, G., Watkins, S. C., Bannykh, S. I., and Aridor, M. (2005). Regulation of Sar1 NH2 terminus by GTP binding and hydrolysis promotes membrane deformation to control COPII vesicle fission. J. Cell Biol. 171, 919–924. doi: 10.1083/jcb.200509095
Bitsikas, V., Corrêa, I. R. Jr., and Nichols, B. J. (2014). Clathrin-independent pathways do not contribute significantly to endocytic flux. eLife 3:e03970. doi: 10.7554/eLife.03970
Bowen, A. B., Bourke, A. M., Hiester, B. G., Hanus, C., and Kennedy, M. J. (2017). Golgi-independent secretory trafficking through recycling endosomes in neuronal dendrites and spines. eLife 6:e27362. doi: 10.7554/eLife.27362
Brandizzi, F., and Barlowe, C. (2013). Organization of the ER-Golgi interface for membrane traffic control. Nat. Rev. Mol. Cell Biol. 14, 382–392. doi: 10.1038/nrm3588
Brown, T. C., Correia, S. S., Petrok, C. N., and Esteban, J. A. (2007). Functional compartmentalization of endosomal trafficking for the synaptic delivery of AMPA receptors during long-term potentiation. J. Neurosci. 27, 13311–13315. doi: 10.1523/JNEUROSCI.4258-07.2007
Caracci, M. O., Fuentealba, L. M., and Marzolo, M. P. (2019). Golgi complex dynamics and its implication in prevalent neurological disorders. Front. Cell Dev. Biol. 7:75. doi: 10.3389/fcell.2019.00075
Casanova, J. E., Wang, X., Kumar, R., Bhartur, S. G., Navarre, J., Woodrum, J. E., et al. (1999). Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol. Biol. Cell 10, 47–61. doi: 10.1091/mbc.10.1.47
Caswell, P. T., Spence, H. J., Parsons, M., White, D. P., Clark, K., Cheng, K. W., et al. (2007). Rab25 associates with α5β1 integrin to promote invasive migration in 3D microenvironments. Dev. Cell 13, 496–510. doi: 10.1016/j.devcel.2007.08.012
Cataldo, A. M., Peterhoff, C. M., Troncoso, J. C., Gomez-Isla, T., Hyman, B. T., and Nixon, R. A. (2000). Endocytic pathway abnormalities precede amyloid β deposition in sporadic Alzheimer’s disease and Down syndrome: differential effects of APOE genotype and presenilin mutations. Am. J. Pathol. 157, 277–286. doi: 10.1016/s0002-9440(10)64538-5
Chen, K. E., Healy, M. D., and Collins, B. M. (2019). Towards a molecular understanding of endosomal trafficking by Retromer and Retriever. Traffic 20, 465–478. doi: 10.1111/tra.12649
Chen, C. C., Schweinsberg, P. J., Vashist, S., Mareiniss, D. P., Lambie, E. J., and Grant, B. D. (2006). RAB-10 is required for endocytic recycling in the Caenorhabditis elegans intestine. Mol. Biol. Cell 17, 1286–1297. doi: 10.1091/mbc.e05-08-0787
Cheng, X. T., Xie, Y. X., Zhou, B., Huang, N., Farfel-Becker, T., and Sheng, Z. H. (2018). Characterization of LAMP1-labeled nondegradative lysosomal and endocytic compartments in neurons. J. Cell Biol. 217, 3127–3139. doi: 10.1083/jcb.201711083
Cho, H. J., Yu, J., Xie, C., Rudrabhatla, P., Chen, X., Wu, J., et al. (2014). Leucine-rich repeat kinase 2 regulates Sec16A at ER exit sites to allow ER-Golgi export. EMBO J. 33, 2314–2331. doi: 10.15252/embj.201487807
Chung, C. G., Kwon, M. J., Jeon, K. H., Hyeon, D. Y., Han, M. H., Park, J. H., et al. (2017). Golgi outpost synthesis impaired by toxic polyglutamine proteins contributes to dendritic pathology in neurons. Cell Rep. 20, 356–369. doi: 10.1016/j.celrep.2017.06.059
Civril, F., Wehenkel, A., Giorgi, F. M., Santaguida, S., Di Fonzo, A., Grigorean, G., et al. (2010). Structural analysis of the RZZ complex reveals common ancestry with multisubunit vesicle tethering machinery. Structure 18, 616–626. doi: 10.1016/j.str.2010.02.014
Clayton, E. L., Milioto, C., Muralidharan, B., Norona, F. E., Edgar, J. R., Soriano, A., et al. (2018). Frontotemporal dementia causative CHMP2B impairs neuronal endolysosomal traffic-rescue by TMEM106B knockdown. Brain 141, 3428–3442. doi: 10.1093/brain/awy284
Coco, S., Raposo, G., Martinez, S., Fontaine, J. J., Takamori, S., Zahraoui, A., et al. (1999). Subcellular localization of tetanus neurotoxin-insensitive vesicle-associated membrane protein (VAMP)/VAMP7 in neuronal cells: evidence for a novel membrane compartment. J. Neurosci. 19, 9803–9812. doi: 10.1523/JNEUROSCI.19-22-09803.1999
Colombo, F., Racchetti, G., and Meldolesi, J. (2014). Neurite outgrowth induced by NGF or L1CAM via activation of the TrkA receptor is sustained also by the exocytosis of enlargeosomes. Proc. Natl. Acad. Sci. U S A 111, 16943–16948. doi: 10.1073/pnas.1406097111
Cooney, J. R., Hurlburt, J. L., Selig, D. K., Harris, K. M., and Fiala, J. C. (2002). Endosomal compartments serve multiple hippocampal dendritic spines from a widespread rather than a local store of recycling membrane. J. Neurosci. 22, 2215–2224. doi: 10.1523/JNEUROSCI.22-06-02215.2002
Copf, T. (2014). Developmental shaping of dendritic arbors in Drosophila relies on tightly regulated intra-neuronal activity of protein kinase A (PKA). Dev. Biol. 393, 282–297. doi: 10.1016/j.ydbio.2014.07.002
Cosson, P., and Letourneur, F. (1994). Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science 263, 1629–1631. doi: 10.1126/science.8128252
Dagda, R. K., Pien, I., Wang, R., Zhu, J., Wang, K. Z., Callio, J., et al. (2014). Beyond the mitochondrion: cytosolic PINK1 remodels dendrites through protein kinase A. J. Neurochem. 128, 864–877. doi: 10.1111/jnc.12494
Day, K. J., Casler, J. C., and Glick, B. S. (2018). Budding yeast has a minimal endomembrane system. Dev. Cell 44, 56.e4–72.e4. doi: 10.1016/j.devcel.2017.12.014
Dell’Orco, J. M., Wasserman, A. H., Chopra, R., Ingram, M. A., Hu, Y. S., Singh, V., et al. (2015). Neuronal atrophy early in degenerative ataxia is a compensatory mechanism to regulate membrane excitability. J. Neurosci. 35, 11292–11307. doi: 10.1523/JNEUROSCI.1357-15.2015
Dozynkiewicz, M. A., Jamieson, N. B., Macpherson, I., Grindlay, J., Van Den Berghe, P. V., Von Thun, A., et al. (2012). Rab25 and CLIC3 collaborate to promote integrin recycling from late endosomes/lysosomes and drive cancer progression. Dev. Cell 22, 131–145. doi: 10.1016/j.devcel.2011.11.008
D’Souza, R. S., Semus, R., Billings, E. A., Meyer, C. B., Conger, K., and Casanova, J. E. (2014). Rab4 orchestrates a small GTPase cascade for recruitment of adaptor proteins to early endosomes. Curr. Biol. 24, 1187–1198. doi: 10.1016/j.devcel.2011.11.008
Esseltine, J. L., Dale, L. B., and Ferguson, S. S. (2011). Rab GTPases bind at a common site within the angiotensin II type I receptor carboxyl-terminal tail: evidence that Rab4 regulates receptor phosphorylation, desensitization, and resensitization. Mol. Pharmacol. 79, 175–184. doi: 10.1124/mol.110.068379
Etoh, K., and Fukuda, M. (2019). Rab10 regulates tubular endosome formation through KIF13A and KIF13B motors. J. Cell Sci. 132:jcs226977. doi: 10.1242/jcs.226977
Eugster, A., Frigerio, G., Dale, M., and Duden, R. (2000). COP I domains required for coatomer integrity, and novel interactions with ARF and ARF-GAP. EMBO J. 19, 3905–3917. doi: 10.1093/emboj/19.15.3905
Evans, A. J., Gurung, S., Wilkinson, K. A., Stephens, D. J., and Henley, J. M. (2017). Assembly, secretory pathway trafficking, and surface delivery of kainate receptors is regulated by neuronal activity. Cell Rep. 19, 2613–2626. doi: 10.1016/j.celrep.2017.06.001
Ferrer, I. (1999). Neurons and their dendrites in frontotemporal dementia. Dement. Geriatr. Cogn. Disord. 10, 55–60. doi: 10.1159/000051214
Fogarty, M. J., Mu, E. W. H., Lavidis, N. A., Noakes, P. G., and Bellingham, M. C. (2017). Motor areas show altered dendritic structure in an amyotrophic lateral sclerosis mouse model. Front. Neurosci. 11:609. doi: 10.3389/fnins.2017.00609
Fogarty, M. J., Mu, E. W., Noakes, P. G., Lavidis, N. A., and Bellingham, M. C. (2016). Marked changes in dendritic structure and spine density precede significant neuronal death in vulnerable cortical pyramidal neuron populations in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Acta Neuropathol. Commun. 4:77. doi: 10.1186/s40478-016-0347-y
Fox, R. M., Hanlon, C. D., and Andrew, D. J. (2010). The CrebA/Creb3-like transcription factors are major and direct regulators of secretory capacity. J. Cell Biol. 191, 479–492. doi: 10.1083/jcb.201004062
Fromme, J. C., Ravazzola, M., Hamamoto, S., Al-Balwi, M., Eyaid, W., Boyadjiev, S. A., et al. (2007). The genetic basis of a craniofacial disease provides insight into COPII coat assembly. Dev. Cell 13, 623–634. doi: 10.1016/j.devcel.2007.10.005
Fujii, S., Kurokawa, K., Inaba, R., Hiramatsu, N., Tago, T., Nakamura, Y., et al. (2020a). Recycling endosomes attach to the trans-side of Golgi stacks in Drosophila and mammalian cells. J. Cell Sci. 133:jcs236935. doi: 10.1242/jcs.236935
Fujii, S., Tago, T., Sakamoto, N., Yamamoto, T., Satoh, T., and Satoh, A. K. (2020b). Recycling endosomes associate with Golgi stacks in sea urchin embryos. Commun. Integr. Biol. 13, 59–62. doi: 10.1080/19420889.2020.1761069
Ginsberg, S. D., Mufson, E. J., Alldred, M. J., Counts, S. E., Wuu, J., Nixon, R. A., et al. (2011). Upregulation of select rab GTPases in cholinergic basal forebrain neurons in mild cognitive impairment and Alzheimer’s disease. J. Chem. Neuroanat. 42, 102–110. doi: 10.1016/j.jchemneu.2011.05.012
Gorrie, G. H., Fecto, F., Radzicki, D., Weiss, C., Shi, Y., Dong, H., et al. (2014). Dendritic spinopathy in transgenic mice expressing ALS/dementia-linked mutant UBQLN2. Proc. Natl. Acad. Sci. U S A 111, 14524–14529. doi: 10.1073/pnas.1405741111
Gosavi, N., Lee, H. J., Lee, J. S., Patel, S., and Lee, S. J. (2002). Golgi fragmentation occurs in the cells with prefibrillar α-synuclein aggregates and precedes the formation of fibrillar inclusion. J. Biol. Chem. 277, 48984–48992. doi: 10.1074/jbc.m208194200
Grant, B. D., and Donaldson, J. G. (2009). Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 10, 597–608. doi: 10.1038/nrm2755
Graveland, G. A., Williams, R. S., and Difiglia, M. (1985). Evidence for degenerative and regenerative changes in neostriatal spiny neurons in Huntington’s disease. Science 227, 770–773. doi: 10.1126/science.3155875
Gray, E. G. (1959a). Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study. J. Anat. 93, 420–433.
Gray, E. G. (1959b). Electron microscopy of synaptic contacts on dendrite spines of the cerebral cortex. Nature 183, 1592–1593. doi: 10.1038/1831592a0
Gromova, K. V., Muhia, M., Rothammer, N., Gee, C. E., Thies, E., Schaefer, I., et al. (2018). Neurobeachin and the kinesin KIF21B are critical for endocytic recycling of NMDA receptors and regulate social behavior. Cell Rep. 23, 2705–2717. doi: 10.1016/j.celrep.2018.04.112
Grote, E., Carr, C. M., and Novick, P. J. (2000). Ordering the final events in yeast exocytosis. J. Cell Biol. 151, 439–452. doi: 10.1083/jcb.151.2.439
Guadagno, N. A., and Progida, C. (2019). Rab GTPases: switching to human diseases. Cells 8:909. doi: 10.3390/cells8080909
Hanus, C., Kochen, L., Tom Dieck, S., Racine, V., Sibarita, J. B., Schuman, E. M., et al. (2014). Synaptic control of secretory trafficking in dendrites. Cell Rep. 7, 1771–1778. doi: 10.1016/j.celrep.2014.05.028
Hasel, P., Mckay, S., Qiu, J., and Hardingham, G. E. (2015). Selective dendritic susceptibility to bioenergetic, excitotoxic and redox perturbations in cortical neurons. Biochim. Biophys. Acta 1853, 2066–2076. doi: 10.1016/j.bbamcr.2014.12.021
Hattula, K., Furuhjelm, J., Tikkanen, J., Tanhuanpaa, K., Laakkonen, P., and Peranen, J. (2006). Characterization of the Rab8-specific membrane traffic route linked to protrusion formation. J. Cell Sci. 119, 4866–4877. doi: 10.1242/jcs.03275
Heider, M. R., and Munson, M. (2012). Exorcising the exocyst complex. Traffic 13, 898–907. doi: 10.1111/j.1600-0854.2012.01353.x
Herms, J., and Dorostkar, M. M. (2016). Dendritic spine pathology in neurodegenerative diseases. Annu. Rev. Pathol. 11, 221–250. doi: 10.1146/annurev-pathol-012615-044216
Hirose, H., Arasaki, K., Dohmae, N., Takio, K., Hatsuzawa, K., Nagahama, M., et al. (2004). Implication of ZW10 in membrane trafficking between the endoplasmic reticulum and Golgi. EMBO J. 23, 1267–1278. doi: 10.1038/sj.emboj.7600135
Ho, W. Y., Tai, Y. K., Chang, J. C., Liang, J., Tyan, S. H., Chen, S., et al. (2019). The ALS-FTD-linked gene product, C9orf72, regulates neuronal morphogenesis via autophagy. Autophagy 15, 827–842. doi: 10.1080/15548627.2019.1569441
Hoffmann, N. A., Dorostkar, M. M., Blumenstock, S., Goedert, M., and Herms, J. (2013). Impaired plasticity of cortical dendritic spines in P301S tau transgenic mice. Acta Neuropathol. Commun. 1:82. doi: 10.1186/2051-5960-1-82
Hoogenraad, C. C., Popa, I., Futai, K., Martinez-Sanchez, E., Wulf, P. S., Van Vlijmen, T., et al. (2010). Neuron specific Rab4 effector GRASP-1 coordinates membrane specialization and maturation of recycling endosomes. PLoS Biol. 8:e1000283. doi: 10.1371/journal.pbio.1000283
Horgan, C. P., Hanscom, S. R., Jolly, R. S., Futter, C. E., and McCaffrey, M. W. (2010). Rab11-FIP3 links the Rab11 GTPase and cytoplasmic dynein to mediate transport to the endosomal-recycling compartment. J. Cell Sci. 123, 181–191. doi: 10.1242/jcs.052670
Horiuchi, H., Lippé, R., McBride, H. M., Rubino, M., Woodman, P., Stenmark, H., et al. (1997). A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell 90, 1149–1159. doi: 10.1016/s0092-8674(00)80380-3
Horton, A. C., and Ehlers, M. D. (2003). Dual modes of endoplasmic reticulum-to-Golgi transport in dendrites revealed by live-cell imaging. J. Neurosci. 23, 6188–6199. doi: 10.1523/JNEUROSCI.23-15-06188.2003
Horton, A. C., and Ehlers, M. D. (2004). Secretory trafficking in neuronal dendrites. Nat. Cell Biol. 6, 585–591. doi: 10.1038/ncb0704-585
Horton, A. C., Racz, B., Monson, E. E., Lin, A. L., Weinberg, R. J., and Ehlers, M. D. (2005). Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron 48, 757–771. doi: 10.1016/j.neuron.2005.11.005
Hsieh, H., Boehm, J., Sato, C., Iwatsubo, T., Tomita, T., Sisodia, S., et al. (2006). AMPAR removal underlies Aβ-induced synaptic depression and dendritic spine loss. Neuron 52, 831–843. doi: 10.1016/j.neuron.2006.10.035
Hu, Y. B., Dammer, E. B., Ren, R. J., and Wang, G. (2015). The endosomal-lysosomal system: from acidification and cargo sorting to neurodegeneration. Transl. Neurodegener. 4:18. doi: 10.1186/s40035-015-0041-1
Hughes, H., Budnik, A., Schmidt, K., Palmer, K. J., Mantell, J., Noakes, C., et al. (2009). Organisation of human ER-exit sites: requirements for the localisation of Sec16 to transitional ER. J. Cell Sci. 122, 2924–2934. doi: 10.1242/jcs.044032
Huotari, J., and Helenius, A. (2011). Endosome maturation. EMBO J. 30, 3481–3500. doi: 10.1038/emboj.2011.286
Jan, Y. N., and Jan, L. Y. (2010). Branching out: mechanisms of dendritic arborization. Nat. Rev. Neurosci. 11, 316–328. doi: 10.1038/nrn2836
Jaworski, T., Lechat, B., Demedts, D., Gielis, L., Devijver, H., Borghgraef, P., et al. (2011). Dendritic degeneration, neurovascular defects and inflammation precede neuronal loss in a mouse model for tau-mediated neurodegeneration. Am. J. Pathol. 179, 2001–2015. doi: 10.1016/j.ajpath.2011.06.025
Jeong, H., Lim, K. M., Kim, K. H., Cho, Y., Lee, B., Knowles, B. C., et al. (2019). Loss of Rab25 promotes the development of skin squamous cell carcinoma through the dysregulation of integrin trafficking. J. Pathol. 249, 227–240. doi: 10.1002/path.5311
Jeyifous, O., Waites, C. L., Specht, C. G., Fujisawa, S., Schubert, M., Lin, E. I., et al. (2009). SAP97 and CASK mediate sorting of NMDA receptors through a previously unknown secretory pathway. Nat. Neurosci. 12, 1011–1019. doi: 10.1038/nn.2362
Jonker, C. T. H., Deo, C., Zager, P. J., Tkachuk, A. N., Weinstein, A. M., Rodriguez-Boulan, E., et al. (2020). Accurate measurement of fast endocytic recycling kinetics in real time. J. Cell Sci. 133:jcs231225. doi: 10.1242/jcs.231225
Kanamori, T., Yoshino, J., Yasunaga, K., Dairyo, Y., and Emoto, K. (2015). Local endocytosis triggers dendritic thinning and pruning in Drosophila sensory neurons. Nat. Commun. 6:6515. doi: 10.1038/ncomms7515
Kelliher, M. T., Saunders, H. A., and Wildonger, J. (2019). Microtubule control of functional architecture in neurons. Curr. Opin. Neurobiol. 57, 39–45. doi: 10.1016/j.conb.2019.01.003
Kelly, E. E., Horgan, C. P., and Mccaffrey, M. W. (2012). Rab11 proteins in health and disease. Biochem. Soc. Trans. 40, 1360–1367. doi: 10.1042/bst20120157
Kennedy, M. J., and Hanus, C. (2019). Architecture and dynamics of the neuronal secretory network. Annu. Rev. Cell Dev. Biol. 35, 543–566. doi: 10.1146/annurev-cellbio-100818-125418
Kiral, F. R., Kohrs, F. E., Jin, E. J., and Hiesinger, P. R. (2018). Rab GTPases and membrane trafficking in neurodegeneration. Curr. Biol. 28, R471–R486. doi: 10.1016/j.cub.2018.02.010
Klapstein, G. J., Fisher, R. S., Zanjani, H., Cepeda, C., Jokel, E. S., Chesselet, M. F., et al. (2001). Electrophysiological and morphological changes in striatal spiny neurons in R6/2 Huntington’s disease transgenic mice. J. Neurophysiol. 86, 2667–2677. doi: 10.1152/jn.2001.86.6.2667
Kobayashi, H., Etoh, K., and Fukuda, M. (2014). Rab35 is translocated from Arf6-positive perinuclear recycling endosomes to neurite tips during neurite outgrowth. Small GTPases 5:e29290. doi: 10.4161/sgtp.29290
Kobayashi, H., and Fukuda, M. (2013). Rab35 establishes the EHD1-association site by coordinating two distinct effectors during neurite outgrowth. J. Cell Sci. 126, 2424–2435. doi: 10.1242/jcs.117846
Kouranti, I., Sachse, M., Arouche, N., Goud, B., and Echard, A. (2006). Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr. Biol. 16, 1719–1725. doi: 10.1016/j.cub.2006.07.020
Kramer, R., Rode, S., and Rumpf, S. (2019). Rab11 is required for neurite pruning and developmental membrane protein degradation in Drosophila sensory neurons. Dev. Biol. 451, 68–78. doi: 10.1016/j.ydbio.2019.03.003
Kulkarni, V. A., and Firestein, B. L. (2012). The dendritic tree and brain disorders. Mol. Cell. Neurosci. 50, 10–20. doi: 10.1016/j.mcn.2012.03.005
Kweon, J. H., Kim, S., and Lee, S. B. (2017). The cellular basis of dendrite pathology in neurodegenerative diseases. BMB Rep. 50, 5–11. doi: 10.5483/bmbrep.2017.50.1.131
Kwon, M. J., Han, M. H., Bagley, J. A., Hyeon, D. Y., Ko, B. S., Lee, Y. M., et al. (2018). Coiled-coil structure-dependent interactions between polyQ proteins and Foxo lead to dendrite pathology and behavioral defects. Proc. Natl. Acad. Sci. U S A 115, E10748–E10757. doi: 10.1073/pnas.1807206115
Larsen, K. E., Schmitz, Y., Troyer, M. D., Mosharov, E., Dietrich, P., Quazi, A. Z., et al. (2006). α-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J. Neurosci. 26, 11915–11922. doi: 10.1523/JNEUROSCI.3821-06.2006
Lazo, O. M., Gonzalez, A., Ascano, M., Kuruvilla, R., Couve, A., and Bronfman, F. C. (2013). BDNF regulates Rab11-mediated recycling endosome dynamics to induce dendritic branching. J. Neurosci. 33, 6112–6122. doi: 10.1523/JNEUROSCI.4630-12.2013
Lei, W., Omotade, O. F., Myers, K. R., and Zheng, J. Q. (2016). Actin cytoskeleton in dendritic spine development and plasticity. Curr. Opin. Neurobiol. 39, 86–92. doi: 10.1002/cm.21280
Leonard, D., Hayakawa, A., Lawe, D., Lambright, D., Bellve, K. D., Standley, C., et al. (2008). Sorting of EGF and transferrin at the plasma membrane and by cargo-specific signaling to EEA1-enriched endosomes. J. Cell Sci. 121, 3445–3458. doi: 10.1242/jcs.031484
Lewerenz, J., and Maher, P. (2015). Chronic glutamate toxicity in neurodegenerative diseases-what is the evidence? Front. Neurosci. 9:469. doi: 10.3389/fnins.2015.00469
Li, X., Sapp, E., Chase, K., Comer-Tierney, L. A., Masso, N., Alexander, J., et al. (2009). Disruption of Rab11 activity in a knock-in mouse model of Huntington’s disease. Neurobiol. Dis. 36, 374–383. doi: 10.1016/j.nbd.2009.08.003
Liazoghli, D., Perreault, S., Micheva, K. D., Desjardins, M., and Leclerc, N. (2005). Fragmentation of the Golgi apparatus induced by the overexpression of wild-type and mutant human tau forms in neurons. Am. J. Pathol. 166, 1499–1514. doi: 10.1016/s0002-9440(10)62366-8
Lin, T., Kao, H. H., Chou, C. H., Chou, C. Y., Liao, Y. C., and Lee, H. H. (2020). Rab11 activation by Ik2 kinase is required for dendrite pruning in Drosophila sensory neurons. PLoS Genet. 16:e1008626. doi: 10.1371/journal.pgen.1008626
Lin, C. H., Li, H., Lee, Y. N., Cheng, Y. J., Wu, R. M., and Chien, C. T. (2015). Lrrk regulates the dynamic profile of dendritic Golgi outposts through the golgin Lava lamp. J. Cell Biol. 210, 471–483. doi: 10.1083/jcb.201411033
Lin, C. H., Tsai, P.-I., Wu, R.-M., and Chien, C.-T. (2010). LRRK2 G2019S mutation induces dendrite degeneration through mislocalization and phosphorylation of tau by recruiting autoactivated GSK3β. J. Neurosci. 30, 13138–13149. doi: 10.1523/JNEUROSCI.1737-10.2010
Lira, M., Arancibia, D., Orrego, P. R., Montenegro-Venegas, C., Cruz, Y., Garcia, J., et al. (2019). The exocyst component Exo70 modulates dendrite arbor formation, synapse density, and spine maturation in primary hippocampal neurons. Mol. Neurobiol. 56, 4620–4638. doi: 10.1007/s12035-018-1378-0
Liu, C., Mei, M., Li, Q., Roboti, P., Pang, Q., Ying, Z., et al. (2017). Loss of the golgin GM130 causes Golgi disruption, Purkinje neuron loss, and ataxia in mice. Proc. Natl. Acad. Sci. U S A 114, 346–351. doi: 10.1073/pnas.1608576114
Luebke, J. I., Weaver, C. M., Rocher, A. B., Rodriguez, A., Crimins, J. L., Dickstein, D. L., et al. (2010). Dendritic vulnerability in neurodegenerative disease: insights from analyses of cortical pyramidal neurons in transgenic mouse models. Brain Struct. Funct. 214, 181–199. doi: 10.1007/s00429-010-0244-2
Magadan, J. G., Barbieri, M. A., Mesa, R., Stahl, P. D., and Mayorga, L. S. (2006). Rab22a regulates the sorting of transferrin to recycling endosomes. Mol. Cell. Biol. 26, 2595–2614. doi: 10.1128/MCB.26.7.2595-2614.2006
Mallard, F., Antony, C., Tenza, D., Salamero, J., Goud, B., and Johannes, L. (1998). Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of shiga toxin B-fragment transport. J. Cell Biol. 143, 973–990. doi: 10.1083/jcb.143.4.973
Martinez-Arca, S., Alberts, P., Zahraoui, A., Louvard, D., and Galli, T. (2000). Role of tetanus neurotoxin insensitive vesicle-associated membrane protein (TI-VAMP) in vesicular transport mediating neurite outgrowth. J. Cell Biol. 149, 889–900. doi: 10.1083/jcb.149.4.889
Martinez-Arca, S., Coco, S., Mainguy, G., Schenk, U., Alberts, P., Bouille, P., et al. (2001). A common exocytotic mechanism mediates axonal and dendritic outgrowth. J. Neurosci. 21, 3830–3838. doi: 10.1523/JNEUROSCI.21-11-03830.2001
Matsuoka, K., Orci, L., Amherdt, M., Bednarek, S. Y., Hamamoto, S., Schekman, R., et al. (1998). COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell 93, 263–275. doi: 10.1016/s0092-8674(00)81577-9
McLauchlan, H., Newell, J., Morrice, N., Osborne, A., West, M., and Smythe, E. (1998). A novel role for Rab5-GDI in ligand sequestration into calthrin-coated pits. Curr. Biol. 8, 34–45. doi: 10.1016/s0960-9822(98)70018-1
Mehraein, P., Yamada, M., and Tarnowska-Dziduszko, E. (1975). Quantitative study on dendrites and dendritic spines in Alzheimer’s disease and senile dementia. Adv. Neurol. 12, 453–458.
Mehta, S. Q., Hiesinger, P. R., Beronja, S., Zhai, R. G., Schulze, K. L., Verstreken, P., et al. (2005). Mutations in Drosophila sec15 reveal a function in neuronal targeting for a subset of exocyst components. Neuron 46, 219–232. doi: 10.1016/j.neuron.2005.02.029
Meldolesi, J. (2011). Neurite outgrowth: this process, first discovered by Santiago Ramon y Cajal, is sustained by the exocytosis of two distinct types of vesicles. Brain Res. Rev. 66, 246–255. doi: 10.1016/j.brainresrev.2010.06.004
Mellman, I. (1996). Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 12, 575–625. doi: 10.1146/annurev.cellbio.12.1.575
Mikhaylova, M., Bera, S., Kobler, O., Frischknecht, R., and Kreutz, M. R. (2016). A dendritic golgi satellite between ERGIC and retromer. Cell Rep. 14, 189–199. doi: 10.1016/j.celrep.2015.12.024
Montegna, E. A., Bhave, M., Liu, Y., Bhattacharyya, D., and Glick, B. S. (2012). Sec12 binds to Sec16 at transitional ER sites. PLoS One 7:e31156. doi: 10.1371/journal.pone.0031156
Moya-Alvarado, G., Gonzalez, A., Stuardo, N., and Bronfman, F. C. (2018). Brain-derived neurotrophic factor (BDNF) regulates Rab5-positive early endosomes in hippocampal neurons to induce dendritic branching. Front. Cell. Neurosci. 12:493. doi: 10.3389/fncel.2018.00493
Murmu, R. P., Li, W., Holtmaat, A., and Li, J. Y. (2013). Dendritic spine instability leads to progressive neocortical spine loss in a mouse model of Huntington’s disease. J. Neurosci. 33, 12997–13009. doi: 10.1523/JNEUROSCI.5284-12.2013
Murthy, M., Garza, D., Scheller, R. H., and Schwarz, T. L. (2003). Mutations in the exocyst component Sec5 disrupt neuronal membrane traffic, but neurotransmitter release persists. Neuron 37, 433–447. doi: 10.1016/s0896-6273(03)00031-x
Nagano, M., Toshima, J. Y., Siekhaus, D. E., and Toshima, J. (2019). Rab5-mediated endosome formation is regulated at the trans-Golgi network. Commun. Biol. 2:419. doi: 10.1038/s42003-019-0670-5
Nakano, I., and Hirano, A. (1987). Atrophic cell processes of large motor neurons in the anterior horn in amyotrophic lateral sclerosis: observation with silver impregnation method. J. Neuropathol. Exp. Neurol. 46, 40–49. doi: 10.1097/00005072-198701000-00004
Neuner, J., Ovsepian, S. V., Dorostkar, M., Filser, S., Gupta, A., Michalakis, S., et al. (2014). Pathological α-synuclein impairs adult-born granule cell development and functional integration in the olfactory bulb. Nat. Commun. 5:3915. doi: 10.1038/ncomms4915
Odley, A., Hahn, H. S., Lynch, R. A., Marreez, Y., Osinska, H., Robbins, J., et al. (2004). Regulation of cardiac contractility by Rab4-modulated β2-adrenergic receptor recycling. Proc. Natl. Acad. Sci. U S A 101, 7082–7087. doi: 10.1073/pnas.0308335101
Park, M., Penick, E. C., Edwards, J. G., Kauer, J. A., and Ehlers, M. D. (2004). Recycling endosomes supply AMPA receptors for LTP. Science 305, 1972–1975. doi: 10.3410/f.1021622.246634
Park, M., Salgado, J. M., Ostroff, L., Helton, T. D., Robinson, C. G., Harris, K. M., et al. (2006). Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron 52, 817–830. doi: 10.1016/j.neuron.2006.09.040
Patino-Lopez, G., Dong, X., Ben-Aissa, K., Bernot, K. M., Itoh, T., Fukuda, M., et al. (2008). Rab35 and its GAP EPI64C in T cells regulate receptor recycling and immunological synapse formation. J. Biol. Chem. 283, 18323–18330. doi: 10.1074/jbc.m800056200
Patt, S., Gertz, H. J., Gerhard, L., and Cervós-Navarro, J. (1991). Pathological changes in dendrites of substantia nigra neurons in Parkinson’s disease: a Golgi study. Histol. Histopathol. 6, 373–380.
Peng, Y., Lee, J., Rowland, K., Wen, Y., Hua, H., Carlson, N., et al. (2015). Regulation of dendrite growth and maintenance by exocytosis. J. Cell Sci. 128, 4279–4292. doi: 10.1242/jcs.174771
Petkovic, M., Jemaiel, A., Daste, F., Specht, C. G., Izeddin, I., Vorkel, D., et al. (2014). The SNARE Sec22b has a non-fusogenic function in plasma membrane expansion. Nat. Cell Biol. 16, 434–444. doi: 10.1038/ncb2937
Pfenninger, K. H. (2009). Plasma membrane expansion: a neuron’s Herculean task. Nat. Rev. Neurosci. 10, 251–261. doi: 10.1038/nrn2593
Pierce, J. P., Mayer, T., and McCarthy, J. B. (2001). Evidence for a satellite secretory pathway in neuronal dendritic spines. Curr. Biol. 11, 351–355. doi: 10.1016/s0960-9822(01)00077-x
Plowey, E. D., Johnson, J. W., Steer, E., Zhu, W., Eisenberg, D. A., Valentino, N. M., et al. (2014). Mutant LRRK2 enhances glutamatergic synapse activity and evokes excitotoxic dendrite degeneration. Biochim. Biophys. Acta 1842, 1596–1603. doi: 10.1016/j.bbadis.2014.05.016
Praschberger, R., Lowe, S. A., Malintan, N. T., Giachello, C. N. G., Patel, N., Houlden, H., et al. (2017). Mutations in membrin/GOSR2 reveal stringent secretory pathway demands of dendritic growth and synaptic integrity. Cell Rep. 21, 97–109. doi: 10.1016/j.celrep.2017.09.004
Puram, S. V., and Bonni, A. (2013). Cell-intrinsic drivers of dendrite morphogenesis. Development 140, 4657–4671. doi: 10.1242/dev.087676
Quassollo, G., Wojnacki, J., Salas, D. A., Gastaldi, L., Marzolo, M. P., Conde, C., et al. (2015). A RhoA signaling pathway regulates dendritic golgi outpost formation. Curr. Biol. 25, 971–982. doi: 10.1016/j.cub.2015.01.075
Raiborg, C., Wenzel, E. M., Pedersen, N. M., Olsvik, H., Schink, K. O., Schultz, S. W., et al. (2015). Repeated ER-endosome contacts promote endosome translocation and neurite outgrowth. Nature 520, 234–238. doi: 10.1038/nature14359
Ren, Y., Yip, C. K., Tripathi, A., Huie, D., Jeffrey, P. D., Walz, T., et al. (2009). A structure-based mechanism for vesicle capture by the multisubunit tethering complex Dsl1. Cell 139, 1119–1129. doi: 10.1016/j.cell.2009.11.002
Richards, P., Didszun, C., Campesan, S., Simpson, A., Horley, B., Young, K. W., et al. (2011). Dendritic spine loss and neurodegeneration is rescued by Rab11 in models of Huntington’s disease. Cell Death Differ. 18, 191–200. doi: 10.1038/cdd.2010.127
Rojas, A. M., Fuentes, G., Rausell, A., and Valencia, A. (2012). The Ras protein superfamily: evolutionary tree and role of conserved amino acids. J. Cell Biol. 196, 189–201. doi: 10.1083/jcb.201103008
Rubino, M., Miaczynska, M., Lippé, R., and Zerial, M. (2000). Selective membrane recruitment of EEA1 suggests a role in directional transport of clathrin-coated vesicles to early endosomes. J. Biol. Chem. 275, 3745–3748. doi: 10.1074/jbc.275.6.3745
Ryglewski, S., Kadas, D., Hutchinson, K., Schuetzler, N., Vonhoff, F., and Duch, C. (2014). Dendrites are dispensable for basic motoneuron function but essential for fine tuning of behavior. Proc. Natl. Acad. Sci. U S A 111, 18049–18054. doi: 10.1073/pnas.1416247111
Salminen, A., and Novick, P. J. (1987). A ras-like protein is required for a post-golgi event in yeast secretion. Cell 49, 527–538. doi: 10.1016/0092-8674(87)90455-7
Saraceno, C., Marcello, E., Di Marino, D., Borroni, B., Claeysen, S., Perroy, J., et al. (2014). SAP97-mediated ADAM10 trafficking from Golgi outposts depends on PKC phosphorylation. Cell Death Dis. 5:e1547. doi: 10.1038/cddis.2014.492
Sato, M., Yoshimura, S., Hirai, R., Goto, A., Kunii, M., Atik, N., et al. (2011). The role of VAMP7/TI-VAMP in cell polarity and lysosomal exocytosis in vivo. Traffic 12, 1383–1393. doi: 10.1111/j.1600-0854.2011.01247.x
Satoh, D., Sato, D., Tsuyama, T., Saito, M., Ohkura, H., Rolls, M. M., et al. (2008). Spatial control of branching within dendritic arbors by dynein-dependent transport of Rab5-endosomes. Nat. Cell Biol. 10, 1164–1171. doi: 10.1038/ncb1776
Schwenk, B. M., Hartmann, H., Serdaroglu, A., Schludi, M. H., Hornburg, D., Meissner, F., et al. (2016). TDP-43 loss of function inhibits endosomal trafficking and alters trophic signaling in neurons. EMBO J. 35, 2350–2370. doi: 10.15252/embj.201694221
Seachrist, J. L., Anborgh, P. H., and Ferguson, S. S. G. (2000). β2-adrenergic receptor internalization, endosomal sorting, and plasma membrane recycling are regulated by rab GTPases. J. Biol. Chem. 275, 27221–27228. doi: 10.1074/jbc.M003657200
Seachrist, J. L., and Ferguson, S. S. (2003). Regulation of G protein-coupled receptor endocytosis and trafficking by Rab GTPases. Life Sci. 74, 225–235. doi: 10.1016/j.lfs.2003.09.009
Semerdjieva, S., Shortt, B., Maxwell, E., Singh, S., Fonarev, P., Hansen, J., et al. (2008). Coordinated regulation of AP2 uncoating from clathrin-coated vesicles by rab5 and hRME-6. J. Cell Biol. 183, 499–511. doi: 10.1083/jcb.200806016
Shorey, M., Stone, M. C., Mandel, J., and Rolls, M. M. (2020). Neurons survive simultaneous injury to axons and dendrites and regrow both types of processes in vivo. Dev. Biol. 465, 108–118. doi: 10.1016/j.ydbio.2020.07.006
Šišková, Z., Justus, D., Kaneko, H., Friedrichs, D., Henneberg, N., Beutel, T., et al. (2014). Dendritic structural degeneration is functionally linked to cellular hyperexcitability in a mouse model of Alzheimer’s disease. Neuron 84, 1023–1033. doi: 10.1016/j.neuron.2014.10.024
Sönnichsen, B., De Renzis, S., Nielsen, E., Rietdorf, J., and Zerial, M. (2000). Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J. Cell Biol. 149, 901–914. doi: 10.1083/jcb.149.4.901
Spang, A. (2013). Retrograde traffic from the Golgi to the endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 5:a013391. doi: 10.1101/cshperspect.a013391
Spencer, B., Kim, C., Gonzalez, T., Bisquertt, A., Patrick, C., Rockenstein, E., et al. (2016). α-Synuclein interferes with the ESCRT-III complex contributing to the pathogenesis of Lewy body disease. Hum. Mol. Genet. 25, 1100–1115. doi: 10.1093/hmg/ddv633
Spires, T. L., Grote, H. E., Garry, S., Cordery, P. M., Van Dellen, A., Blakemore, C., et al. (2004). Dendritic spine pathology and deficits in experience-dependent dendritic plasticity in R6/1 Huntington’s disease transgenic mice. Eur. J. Neurosci. 19, 2799–2807. doi: 10.1111/j.0953-816x.2004.03374.x
Spires, T. L., Meyer-Luehmann, M., Stern, E. A., Mclean, P. J., Skoch, J., Nguyen, P. T., et al. (2005). Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J. Neurosci. 25, 7278–7287. doi: 10.1523/JNEUROSCI.1879-05.2005
Spires-Jones, T. L., Meyer-Luehmann, M., Osetek, J. D., Jones, P. B., Stern, E. A., Bacskai, B. J., et al. (2007). Impaired spine stability underlies plaque-related spine loss in an Alzheimer’s disease mouse model. Am. J. Pathol. 171, 1304–1311. doi: 10.2353/ajpath.2007.070055
Stenmark, H. (2009). Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 10, 513–525. doi: 10.1038/nrm2728
Stenmark, H., Vitale, G., Ullrich, O., and Zerial, M. (1995). Rabaptin-5 Is a direct effector of the small Gtpase Rab5 in endocytic membrane-fusion. Cell 83, 423–432. doi: 10.1016/0092-8674(95)90120-5
Suda, Y., Kurokawa, K., and Nakano, A. (2017). Regulation of ER-Golgi transport dynamics by GTPases in budding yeast. Front. Cell Dev. Biol. 5:122. doi: 10.3389/fcell.2017.00122
Südhof, T. C., and Rothman, J. E. (2009). Membrane fusion: grappling with SNARE and SM proteins. Science 323, 474–477. doi: 10.1126/science.1161748
Sztul, E., and Lupashin, V. (2009). Role of vesicle tethering factors in the ER-Golgi membrane traffic. FEBS Lett. 583, 3770–3783. doi: 10.1016/j.febslet.2009.10.083
Taguchi, T. (2013). Emerging roles of recycling endosomes. J. Biochem. 153, 505–510. doi: 10.1093/jb/mvt034
Tanigawa, G., Orci, L., Amherdt, M., Ravazzola, M., Helms, J. B., and Rothman, J. E. (1993). Hydrolysis of bound GTP by ARF protein triggers uncoating of Golgi-derived COP-coated vesicles. J. Cell Biol. 123, 1365–1371. doi: 10.1083/jcb.123.6.1365
Taylor, C. A., Yan, J., Howell, A. S., Dong, X., and Shen, K. (2015). RAB-10 regulates dendritic branching by balancing dendritic transport. PLoS Genet. 11:e1005695. doi: 10.1371/journal.pgen.1005695
TerBush, D. R., Maurice, T., Roth, D., and Novick, P. (1996). The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 15, 6483–6494. doi: 10.1002/j.1460-2075.1996.tb01039.x
Ullrich, O., Reinsch, S., Urbé, S., Zerial, M., and Parton, R. G. (1996). Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 135, 913–924. doi: 10.1083/jcb.135.4.913
Umeda, T., Ramser, E. M., Yamashita, M., Nakajima, K., Mori, H., Silverman, M. A., et al. (2015). Intracellular amyloid β oligomers impair organelle transport and induce dendritic spine loss in primary neurons. Acta Neuropathol. Commun. 3:51. doi: 10.1186/s40478-016-0273-z
Urbina, F. L., Gomez, S. M., and Gupton, S. L. (2018). Spatiotemporal organization of exocytosis emerges during neuronal shape change. J. Cell Biol. 217, 1113–1128. doi: 10.1083/jcb.201709064
van Dis, V., Kuijpers, M., Haasdijk, E. D., Teuling, E., Oakes, S. A., Hoogenraad, C. C., et al. (2014). Golgi fragmentation precedes neuromuscular denervation and is associated with endosome abnormalities in SOD1-ALS mouse motor neurons. Acta Neuropathol. Commun. 2:38. doi: 10.1186/2051-5960-2-38
Vega, I. E., and Hsu, S. C. (2001). The exocyst complex associates with microtubules to mediate vesicle targeting and neurite outgrowth. J. Neurosci. 21, 3839–3848. doi: 10.1523/jneurosci.21-11-03839.2001
von Zastrow, M., and Williams, J. T. (2012). Modulating neuromodulation by receptor membrane traffic in the endocytic pathway. Neuron 76, 22–32. doi: 10.1016/j.neuron.2012.09.022
Wandinger-Ness, A., and Zerial, M. (2014). Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb. Perspect. Biol. 6:a022616. doi: 10.1101/cshperspect.a022616
Wang, Z., Edwards, J. G., Riley, N., Provance, D. W. Jr., Karcher, R., Li, X. D., et al. (2008). Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell 135, 535–548. doi: 10.1016/j.cell.2008.09.057
Wang, L., Liang, Z., and Li, G. (2011). Rab22 controls NGF signaling and neurite outgrowth in PC12 cells. Mol. Biol. Cell 22, 3853–3860. doi: 10.1091/mbc.e11-03-0277
Wang, X., Kumar, R., Navarre, J., Casanova, J. E., and Goldenring, J. R. (2000). Regulation of vesicle trafficking in madin-darby canine kidney cells by Rab11a and Rab25. J. Biol. Chem. 275, 29138–29146. doi: 10.1074/jbc.m004410200
Wang, B., Stanford, K. R., and Kundu, M. (2020). ER-to-golgi trafficking and its implication in neurological diseases. Cells 9:408. doi: 10.3390/cells9020408
Wang, Y., Zhang, H., Shi, M., Liou, Y. C., Lu, L., and Yu, F. (2017). Sec71 functions as a GEF for the small GTPase Arf1 to govern dendrite pruning of Drosophila sensory neurons. Development 144, 1851–1862. doi: 10.1242/dev.146175
Weigert, R., Yeung, A. C., Li, J., and Donaldson, J. G. (2004). Rab22a regulates the recycling of membrane proteins internalized independently of clathrin. Mol. Biol. Cell 15, 3758–3770. doi: 10.1091/mbc.e04-04-0342
Welz, T., Wellbourne-Wood, J., and Kerkhoff, E. (2014). Orchestration of cell surface proteins by Rab11. Trends Cell Biol. 24, 407–415. doi: 10.1016/j.tcb.2014.02.004
Yap, C. C., Digilio, L., Mcmahon, L. P., Garcia, A. D. R., and Winckler, B. (2018). Degradation of dendritic cargos requires Rab7-dependent transport to somatic lysosomes. J. Cell Biol. 217, 3141–3159. doi: 10.1083/jcb.201711039
Ye, B., Zhang, Y., Song, W., Younger, S. H., Jan, L. Y., and Jan, Y. N. (2007). Growing dendrites and axons differ in their reliance on the secretory pathway. Cell 130, 717–729. doi: 10.1016/j.cell.2007.06.032
Yudowski, G. A., Puthenveedu, M. A., Henry, A. G., and Von Zastrow, M. (2009). Cargo-mediated regulation of a rapid Rab4-dependent recycling pathway. Mol. Biol. Cell 20, 2774–2784. doi: 10.1091/mbc.e08-08-0892
Zhang, X. M., Ellis, S., Sriratana, A., Mitchell, C. A., and Rowe, T. (2004). Sec15 is an effector for the Rab11 GTPase in mammalian cells. J. Biol. Chem. 279, 43027–43034. doi: 10.1074/jbc.m402264200
Zhang, H., Wang, Y., Wong, J. J., Lim, K. L., Liou, Y. C., Wang, H., et al. (2014). Endocytic pathways downregulate the L1-type cell adhesion molecule neuroglian to promote dendrite pruning in Drosophila. Dev. Cell 30, 463–478. doi: 10.1016/j.devcel.2014.06.014
Zhen, Y., and Stenmark, H. (2015). Cellular functions of Rab GTPases at a glance. J. Cell Sci. 128, 3171–3176. doi: 10.1242/jcs.166074
Zheng, Y., Wildonger, J., Ye, B., Zhang, Y., Kita, A., Younger, S. H., et al. (2008). Dynein is required for polarized dendritic transport and uniform microtubule orientation in axons. Nat. Cell Biol. 10, 1172–1180. doi: 10.1038/ncb1777
Zhou, W., Chang, J., Wang, X., Savelieff, M. G., Zhao, Y., Ke, S., et al. (2014). GM130 is required for compartmental organization of dendritic golgi outposts. Curr. Biol. 24, 1227–1233. doi: 10.1016/j.cub.2014.04.008
Keywords: plasma membrane turnover, dendritic pathology, neurodegenerative diseases, Rab GTPases, dendritic secretory pathway, dendritic endocytic pathway
Citation: Chung CG, Park SS, Park JH and Lee SB (2020) Dysregulated Plasma Membrane Turnover Underlying Dendritic Pathology in Neurodegenerative Diseases. Front. Cell. Neurosci. 14:556461. doi: 10.3389/fncel.2020.556461
Received: 28 April 2020; Accepted: 03 September 2020;
Published: 06 October 2020.
Edited by:
Peter Soba, University Medical Center Hamburg-Eppendorf, GermanyReviewed by:
Jill Wildonger, University of Wisconsin-Madison, United StatesJay Z. Parrish, University of Washington, United States
Copyright © 2020 Chung, Park, Park and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sung Bae Lee, sblee@dgist.ac.kr
† These authors have contributed equally to this work
 Chang Geon Chung
Chang Geon Chung Sung Soon Park
Sung Soon Park Jeong Hyang Park†
Jeong Hyang Park†  Sung Bae Lee
Sung Bae Lee