Abstract
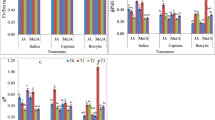
Jasmonates (JAs) are a rising class of lipid-derived signalling molecules with varied functions ranging from induction of abiotic and biotic stress-responsive genes to regulation of plant growth and development under natural condition. The rationale of present study was to investigate the nutritional importance of JAs in inducing and triggering accumulation of carbohydrates, sugars, nutritional pigments and vitamins in three varieties of Brassica oleracea L. (variety botrytis, capitata, italica) edible heads. Exogenous application of Jasmonic acid (JA) and methyl ester of Jasmonic acid (Me–JA), made edible heads of var. botrytis, capitata and italica nutritionally more accrete as compared to control untreated edible heads. Results showed that the treatment of JA and Me-JA enhanced growth of edible heads in terms of head diameter, head length, fresh weight, dry weight, moisture content, total protein, total starch, total carbohydrates, total soluble sugar, non-reducing sugar, vitamins C, A, E and B2 in var. botrytis but at the same time dramatic decline in reducing sugar, beta-carotene and lycopene was seen. In var. capitata certain reduction in vitamin E accumulation was recorded by the exogenous application of JA and Me–JA while overall growth of edible heads was accelerated which could also be supported by enhanced nutritional and productivity level. In variety italica, JAs reduced total starch and vitamin C, but overall growth was surged along with productivity. Both JA and Me-JA could be attributed to induce growth and enhance the nutritional potential of edible heads in three varieties of Brassica oleracea as compared to their untreated control edible heads. From the present results, it has been suggested that these two eco-friendly oxylipins can be further explored for enhancing the growth and nutritional value of these vegetables by exogenous application of JAs in μM to pM concentration.






Similar content being viewed by others
References
Abbaspour H, Rezaei H (2014) Effects of salicylic acid and jasmonic acid on hill reaction and photosynthetic pigment ( DracocephalummoldavicaL.) in different levels of drought stress. IJABBR 2:2850–2859
Avanci NC, Luche DD, Goldman GH, Goldman MHS (2010) Jasmonates are phytohormones with multiple functions, including plant defense and reproduction. Genet Mol Res 9:484–505
Barros, (2006) Total phenols, ascorbic acid, b-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem 103:413–419
Bayfield RF, Cole ER (1980) Colorimetric estimation of vitamin A with trichloroacetic acid. Methods Enzymol 67:180–195
Broeckling CD, Huhman DV, Farag MA, Smith JT, May GD, Mendes P (2005) Metabolic profiling of Medicagotruncatula cell cultures reveals the effects of biotic and abiotic elicitors on metabolism. J Exp Bot 56:323–336
Browse J (2009) Jasmonate: preventing them aizetassel from getting in touch with his feminine side. Sci Signal 2:e9
Chen C, Letnik I, Hacham Y, Dobrev P, Ben-Daniel BH, Vanková R (2014) Ascorbate peroxidase 6 protects Arabidopsis desiccating and germinating seeds from stress and mediates cross talk between reactive oxygen species, abscisic acid, and auxin. Plant Physiol 166:370–383
Chinoy JJ, Singh YD, Gurumurthi K (1976) The role of ascorbic acid in growth, differentiation and metabolism of plants. J Plant Physiol 22:122
Cipollini D (2010) Constitutive expression of methyl jasmonates inducible responses delays reproduction and constrains fitness responses to nutrients in Arabidopsis thaliana. Evol Ecol 24:59–68
Creelman RA, Mullet JE (1997) Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol. Plant Mol Biol 48:355–381
Devoto A, Ellis C, Magusin A, Chang HS, Chilcott C, Zhu T (2005) Expression profiling reveals COI1 to be a key regulator of genes involved in wound- and methyl jasmonate-induced secondary metabolism, defence, and hormone interactions. Plant Mol Biol 58:497–513
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Calometric method for determination of sugars and related substances. Anal Chem 28:350–356
Frenke KC, Jänkänpää HJ, SkogströmDall’Osto OL, Ågren J (2009) Improper excess light energy dissipation in Arabidopsis results in a metabolic reprogramming. BMC Plant Biol 9:12–28
Geyter N, Gholami A, Goormachtig S, Goossens A (2012) Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends Plant Sci 17:349–359
GhasemiPirbalouti A, Sajjadi SE, Parang K (2014) A review (research and patents) on jasmonic acid and its derivatives. Arch Pharm 347:229–239
Hanik N, Gomez S, Best M, Schueller M, Orians CM, Ferrieri RA (2010) Partitioning of new carbon as 11C inNicotiana tabacum reveals insight into methyl jasmonate induced changes in metabolism. J Chem Ecol 36:1058–1067
Ibrahim MH (2011) The relationship between phenolics and flavonoids production with total non structural carbohydrate and photosynthetic rate in labisiapumilabenth. under high CO2 and nitrogen fertilization. Molecules 16:162–174
Jubany-Marı T, Prinsen E, Munne-Bosch S, Alegre L (2010) The timing of methyl jasmonate, hydrogen peroxide and ascorbate accumulation during water deficit and subsequent recovery in the Mediterranean shrub Cistus albidus L. Environ Exp Bot 69:47–55
Jung C, Lyou SH, Yeu S, Kim MA, Rhee S, Lee JS, Kim DCMY, Cheong JJ (2007) Microarray-based screening of jasmonate-responsive genes in Arabidopsis thaliana. Plant Cell Rep 26:1053–1063
Kobayashi H, Yanaka M, Ikeda TM (2010) Exogenous methyl jasmonate alters trichome density on leaf surfaces of Rhodes grass (ChlorisgayanaKunth). J Plant Growth Regul 29:506–511
Koo AJK, Howe GA (2012) Catabolism and deactivation of the lipidhormonejasmonoyL-isoleucine. Front Plant Sci 3:19
Kumar H, Sharma D, Kumar V (2012) Nickel-induced oxidative stress and role of antioxidant defense in barley roots and leaves. Int J Environ Biol 2:121–128
Loewus FA (1952) Improvement in the anthrone method for determination of carbohydrates. Anal Chem 2:219
Lowry H, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein estimation with folin-phenol reagent. J Boil Chem 193:265
Mabood F, Zhou X, Lee KD, Smith DL (2006) Methyl jasmonate, alone or in combination with genistein, and Bradyrhizobium japonicum increases soybean (Glycine max L.) plant dry matter production and grain yield under short season conditions. Field Crop Res 95:412–419
Moreno JE, Tao Y, Chory J, Ballare CL (2009) Ecological modulation of plant defense via phytochrome control of jasmonates sensitivity. Proc Natl Acad Sci USA 106:4935–4940
Mudaser MA et al (2018) Jasmonic acid ameliorates alkaline stress by improving growth performance, ascorbate glutathione cycle and glyoxylase system in maize seedling. Sci Rep 8:2831
Nafie E, Hathout T, Al S, Al M (2011) Jasmonic acid elicits oxidative defense and detoxification systems in Cucumismelo L. cells. Braz J Plant Physiol 23:161–174
Noir S, Bömer M, Takahashi N, Ishida T, Tsui TL, Balbi V, Shanahan H, Sugimoto K, Devoto A (2013) Jasmonate controls leaf growth by repressing cell proliferation and the onset of endoreduplication while maintaining a potential stand-by mode. Plant Physiol 161:1930–1951
Pauwels L, Morreel K, de Witte E, Lammertyn F, van Montagu M, Boerjan W (2008) Mapping methyl jasmonate-mediated transcriptional reprogramming of metabolism and cell cycle progression in cultured Arabidopsis cells. Proc Natl Acad Sci USA 105:1380–1385
Pelacho AM, Mingo-Castel AM (1991) Jasmonic acid induces tuberization of potato stolons cultured in vitro. Plant Physiol 97:1253–1255
Piotrowska A, Bajguz A, Czerpak R, Kot K (2010) Changes in the growth, chemical composition, and antioxidant activity in theaquatic plant Wolffiaarrhiza (L.) Wimm. (Lemnaceae) exposed to jasmonic acid. J Plant Growth Regul 29:53–62
Reyes-Díaz M et al (2016) Methyl jasmonate: An alternative for improving the quality and health properties of fresh fruits. Molecules 6:567
Rohwer CL, Erwin JE (2008) Horticultural applications of jasmonates: a review. J Hortic Sci Biotechnol 83:283–304
Rosenberg HR (1992) Chemistry and physiology of vitamins. Inter Science Publishers Inc., New York
Sirhindi G, Mir MA, Abd-Allah EF, Ahmad P, Gucel S (2016) Jasmonic acid modulates the physio-biochemical attributes, antioxidant enzyme activity, and gene expression in Glycine max under nickel toxicity. Front Plant Sci 7:591
Soares AM, Souza TF, Jacinto T, Machado OLT (2010) Effect of methyl jasmonate on antioxidative enzyme activities and on the contents of ROS and H2O2 in Ricinus communis leaves. Braz J Plant Physiol 22:151–158
Sorial ME, El-Gamal SM, Gendy AA (2010) Response of sweet basil to jasmonic acid application in relation to different water supplies. Bio Res 7:39–47
Tsukada K, Takahashi K, Nabeta K (2010) Biosynthesis of jasmonic acid in a plant pathogenic fungus, Lasiodiplodia theobromae. Phytochemistry 71:2019–2023
Tung P, Hooker TS, Tampe PA, Reid DM, Thorpe TA (1996) Jasmonic acid: effects on growth and development of isolated tomato roots cultured in vitro. Int J Plant Sci 157:713–721
Wasternack C, Goetz S, Hellwege A, Forner S, Strnad M, Hause B (2012) Another JA/COI1-independent role of OPDA detected in tomato embryo development. Plant Signal Behav 7:1349–1353
Wasternack C, Kombrink E (2010) Jasmonates: structural requirements for lipid-derived signals active in plant stress responses and development. ACS Chem Biol 5:63–77
Wu H, Wu X, Li Z, Duan L, Zhang M (2012) Physiological evaluation of drought stress tolerance and recovery in cauliflower (Brassica oleracea L.) seedlings treated with methyl jas-monate and coronatine. J Plant Growth Regul 31:113–123
Yan Y, Borrego E, Kolomiets MV (2013) Jasmonate biosynthesis, perception and function in plant development and stress response. Lipid Metabolism 16:393–442
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sirhindi, G., Kaushik, S., Mushtaq, R. et al. Jasmonates induce growth and modulation in pigments and vitamins of Brassica oleracea var. capitata, italica and botrytis edible heads (foliage/inflorescence). Braz. J. Bot 43, 705–719 (2020). https://doi.org/10.1007/s40415-020-00652-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-020-00652-1