Trace and Rare Earth Elements, and Sr Isotopic Compositions of Fluorite from the Shihuiyao Rare Metal Deposit, Inner Mongolia: Implication for Its Origin
Abstract
:1. Introduction
2. Geological Setting and Mineralization
3. Samples and Analytical Methods
3.1. Samples
3.2. Whole-Rock Trace-Elements Analyses
3.3. Trace-Elements and Sr Isotopes in Fluorite
4. Results
4.1. Trace Elements
4.2. REE and Y
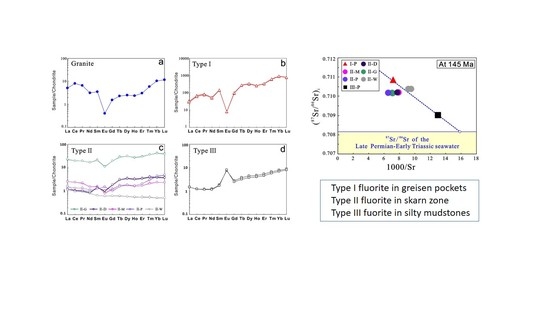
4.3. Fluorite Sr Isotopic Compositions
5. Discussion
5.1. Origin of Fluorite Formation
5.1.1. Type I Fluorites
5.1.2. Type II Fluorites
5.1.3. Type III fluorites
5.2. Fluid Condition
5.3. Source of Sr
5.4. Implication for the Nb-Ta Mineralization
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sasmaz, A.; Yavuz, F.; Sağıroğlu, A.; Akgül, B. Geochemical patterns of the Akdağmadeni (Yozgat, Central Turkey) fluorite deposits and implications. J. Asian Earth Sci. 2005, 24, 469–479. [Google Scholar] [CrossRef]
- Sasmaz, A.; Önal, A.; Sagıroğlu, A.; Önal, M.; Akgül, B. Origin and nature of the mineralizing fluids of thrust zone fluorites in Celikhan (Adıyaman, Eastern Turkey): A geochemical approach. Geochem. J. 2005, 39, 131–139. [Google Scholar] [CrossRef]
- Sasmaz, A.; Yavuz, F. REE geochemistry and fluid inclusion studies of fluorite deposits from the Yaylagzü area (Yıldızeli-Sivas) in central Turkey. Neues Jahrb. Geol. Palaontol. Abh. 2007, 183, 215–226. [Google Scholar]
- Dill, H.G.; Hansen, B.T.; Weber, B. REE contents, REE minerals and Sm/Nd isotopes of granite- and unconformity-related fluorite mineralization at the western edge of the Bohemian massif: With special reference to the Nabburg-Wölsendorf District, SE Germany. Ore Geol. Rev. 2011, 40, 132–148. [Google Scholar] [CrossRef]
- Souissi, F.; Souissi, R.; Dandurand, J.L. The Mississippi valley type (MVT) fluorite ore at Jebel Stah (Zaghouan District, northeastern Tunisia): Contribution of REE and Sr isotope geochemistries to the genetic model. Ore Geol. Rev. 2010, 37, 15–30. [Google Scholar] [CrossRef]
- Ehya, F. Variation of mineralizing fluids and fractionation of REE during the emplacement of the vein-type fluorite deposit at Bozijan, Markazi Province, Iran. J. Geochem. Explor. 2012, 112, 93–106. [Google Scholar] [CrossRef]
- Akgul, B. Geochemical associations between fluorite mineralization and A-type shoshonitic magmatism in the Keban-Elazig area, East Anatolia, Turkey. J. Afr. Earth Sci. 2015, 111, 222–230. [Google Scholar] [CrossRef]
- Azizi, M.R.; Abedini, A.; Alipour, S.; Niroomand, S.; Sasmaz, A.; Talaei, B. Rare earth element geochemistry and tetrad effect in fluorites: A case study from the QahrAbad deposit, Iran. Neues Jahrb. Geol. Palaontol. Abh. 2017, 283, 255–273. [Google Scholar] [CrossRef]
- Pei, Q.; Zhang, S.T.; Santosh, M.; Cao, H.W.; Zhang, W.; Hu, X.K.; Wang, L. Geochronology, geochemistry, fluid inclusion and C, O and Hf isotope compositions of the Shuitou fluorite deposit, Inner Mongolia, China. Ore Geol. Rev. 2017, 83, 174–190. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Ling, H.F.; Jiang, S.Y.; Shen, W.Z.; Fan, H.H.; Ni, P. Trace element and Sr-Nd isotope geochemistry of fluorite from the Xiangshan uranium deposit southeast China. Econ. Geol. 2006, 101, 1613–1622. [Google Scholar] [CrossRef]
- Uras, Y.; Nikiforov, A.V.; Oner, F.; Parlak, O. Geochemistry and Nd, Sr Isotopes of the Pohrenk Fluorites (Kırsehir-Turkey). Geochem. Int. 2017, 55, 263–281. [Google Scholar] [CrossRef]
- Sallet, R.; Moritz, R.; Fontiginie, D. Fluorite 87Sr/86Sr and REE constraints on fluid-melt relations, crystallization time span and DSr of evolved high-silica granites, Tabuleiro granites, Santa Catarina, Brazil. Chem. Geol. 2000, 164, 81–92. [Google Scholar] [CrossRef]
- Sallet, R.; Moritz, R.; Fontiginie, D. The use of vein fluorite as probe for paleofluid REE and Sr-Nd isotope geochemistry: The Santa Catarina Fluorite District, Southern Brazil. Chem. Geol. 2005, 223, 227–248. [Google Scholar] [CrossRef]
- Sánchez, V.; Cardellach, E.; Corbella, M.; Vindel, E.; Martín-Crespo, T.; Boyce, A.J. Variability in fluid sources in the fluorite deposits from Asturias (N Spain): Further evidences from REE, radiogenic (Sr, Sm, Nd) and stable (S, C, O) isotope data. Ore Geol. Rev. 2010, 37, 87–100. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Fan, H.R.; Yang, K.F.; Hu, F.F.; Wang, K.Y.; Chen, P.K.; Yang, Y.H.; Yang, Z.F.; Wang, Q.W. Mesoproterozoic and Paleozoic hydrothermal metasomatism in the giant Bayan Obo REE-Nb-Fe deposit: Constrains from trace elements and Sr-Nd isotope of fluorite and preliminary thermodynamic calculation. Precambrian Res. 2018, 311, 228–246. [Google Scholar] [CrossRef]
- Zhu, J.Z.; Lu, X.C.; Yao, Z.W. Geological characteristics and metallogenic regularity of rare metal deposit in Shihuiyao, Inner Mongolia. Miner. Explor. 2013, 4, 635–641. [Google Scholar]
- Sun, Y.; Wang, R.J.; Li, J.K.; Zhao, Z. 40Ar-39Ar dating of the muscovite and regional exploration prospect of Shihuiyao rubidium-multi-metal deposit, Selenhot, Inner Mongolia. Geol. Rev. 2015, 61, 463–468, (In Chinese with English Abstract). [Google Scholar]
- Duan, X.Z.; Shi, H.; Tan, K.X.; Xie, Y.S.; Chen, L.; Han, S.L.; Hu, Y.; Feng, Z.G.; Zhang, Y.Q.; Guo, H.F.; et al. Geology and genesis of the granitic niobium-tantalum-rubidium deposit in Shihuiyao area, Inner Mongolia. Geol. Resour. 2016, 25, 32–40. (In Chinese) [Google Scholar]
- Liu, Y.J.; Li, W.; Feng, Z.P.; Wen, Q.B.; Neubauer, F.; Liang, C.Y. A review of the Paleozoic tectonics in the eastern part of Central Asian Orogenic Belt. Gondwana Res. 2017, 43, 123–148. [Google Scholar] [CrossRef]
- Mao, J.W.; Xie, G.Q.; Zhang, Z.H.; Li, X.F.; Wang, Y.T.; Zhang, C.Q.; Li, Y.F. Mesozoic large-scale metallogenic pulses in North China and corresponding geodynamic settings. Acta Petrol. Sin. 2005, 21, 169–188. [Google Scholar]
- Wu, F.Y.; Sun, D.Y.; Ge, W.C.; Zhang, Y.B.; Grant, M.L.; Wilde, S.A.; Jahn, B.M. Geochronology of the Phanerozoic granitoids in northeastern China. J. Asian Earth Sci. 2011, 41, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Zhou, J.B.; Wang, B.; Cao, J.L. The final collision of the CAOB: Constraint from the zircon U-Pb dating of the Linxi Formation, Inner Mongolia. Geosci. Front. 2015, 6, 211–225. [Google Scholar] [CrossRef]
- Zhai, D.X.; Zhang, Y.S.; Tian, S.G.; Wu, F.M.; Xing, E.Y.; Wang, M.; Shi, L.Z.; Wang, Z.Z. Sedimentary environment and evolution of the Upper Permian Linxi Formation in Linxi area, Inner Mongolia. J. Palaeogeogr. 2015, 17, 359–370. (In Chinese) [Google Scholar]
- Gao, J.F.; Lu, J.J.; Lai, M.Y.; Lin, Y.P.; Pu, W. Analysis of trace elements in rock samples using HR-ICPMS. J. Nanjing Univ. (Nat. Sci.) 2003, 39, 844–850. (In Chinese) [Google Scholar]
- Govindaraju, K. Compilation of working values and sample description for 383 geostandards. Geostandards Newsletter 1994, 18, 1–158. [Google Scholar] [CrossRef]
- Boynton, W.V. Geochemistry of the rare earth elements: Meteorite studies. In Rare Earth Element Geochemistry; Henderson, P., Ed.; Elsevier: New York, NY, USA, 1984. [Google Scholar]
- Bau, M.; Dulski, P. Comparative study of yttrium and rare-earth element behaviours in fluorine-rich hydrothermal fluids. Contrib. Mineral. Petrol. 1995, 119, 213–223. [Google Scholar] [CrossRef]
- Korte, C.; Kozur, H.W.; Bruchschen, P.; Veizer, J. Strontium isotope evolution of Late Permian and Triassic seawater. Geochim. Cosmochim. Acta 2003, 67, 47–62. [Google Scholar] [CrossRef]
- Nebel, O.; Scherer, E.E.; Mezger, K. Evaluation of the 87Rb decay constant by age comparison against the U-Pb system. Earth Planet. Sci. Lett. 2011, 301, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Michard, A. Rare earth element systematics in hydrothermal fluids. Geochim. Cosmochim. Acta 1989, 53, 745–750. [Google Scholar] [CrossRef]
- Schwinn, G.; Markl, G. REE systematics in hydrothermal fluorite. Chem. Geol. 2005, 216, 225–248. [Google Scholar] [CrossRef]
- Wood, S.A. The aqueous geochemistry of the rare earth elements and yttrium. Review of available low temperature data for inorganic complexes and the inorganic REE speciation of natural waters. Chem. Geol. 1990, 82, 159–186. [Google Scholar] [CrossRef]
- Wood, S.A. The aqueous geochemistry of the rare earth elements and yttrium. Theoretical predictions of speciation in hydrothermal solutions to 350 °C at saturation water vapor pressure. Chem. Geol. 1990, 88, 99–125. [Google Scholar] [CrossRef]
- Haas, J.R.; Shock, E.L.; Sassani, D. Rare earth in hydrothermal systems: Estimates of standard partial molal thermodynamic of aqueous complexes of rare earth elements at high pressures and temperatures. Geochim. Cosmochim. Acta 1995, 21, 4329–4350. [Google Scholar] [CrossRef]
- Möller, P. REE fractionation in hydrothermal fluorite and calcite. In Source, Transport and Deposition of Metals; Pagel, M., Leroy, J., Eds.; Balkema: Rotterdam, The Netherlands, 1991; pp. 91–94. [Google Scholar]
- Lüders, V. Formation of hydrothermal fluorite deposits of the Harz Mountains, Germany. In Source, Transport and Deposition of Metals; Pagel, M., Leroy, J.L., Eds.; Balkema: Rotterdam, The Netherlands, 1991; pp. 325–328. [Google Scholar]
- Borges, R.M.K.; Villas, R.N.N.; Fuzikawa, K.; Dall’Agnol, R.; Pimenta, M.A. Phase separation, fluid mixing, and origin of the greisens and potassic episyenite associated with the gua boa pluton, pitinga tin province, amazonian craton, Brazil. J. S. Am. Earth Sci. 2009, 27, 161–183. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Wang, J.B.; Wang, Y.L.; Cheng, X.Y.; He, P.; Fu, Q.B.; Li, S.T. Characteristics of greisen inclusions in alkali feldspar granite of Yaogangxian tungsten deposit. Miner. Depos. 2013, 32, 533–544. (In Chinese) [Google Scholar]
- Dong, Y.C. Characteristics of greisenization of granite-type W-Sn deposit in Limu of Guangxi. Miner. Resour. Geol. 2014, 28, 699–706. (In Chinese) [Google Scholar]
- Parekh, P.P.; Möller, P.; Dulski, P. Distribution of trace elements between carbonate and non-carbonate phases of limestones. Earth Planet. Sci. Lett. 1977, 34, 39–50. [Google Scholar] [CrossRef]
- Denis, E.; Dabard, M.P. Sandstone petrography and geochemistry of late proterozoic sediments of the armorican massif (France)-A key to basin development during the cadomian orogeny. Precambrian Res. 1989, 42, 189–206. [Google Scholar] [CrossRef]
- Maravelis, A.; Zelilidis, A. Petrography and geochemistry of the late Eocene-early Oligocene submarine fans and shelf deposits on Lemnos Island, NE Greece. Implications for provenance and tectonic setting. Geol. J. 2010, 45, 412–433. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, S. Geochemistry of Permo-Triassic mudstone of the Satpura Gondwana basin, central India: Clues for provenance. Chem. Geol. 2010, 277, 78–100. [Google Scholar] [CrossRef]
- Xie, Q.F.; Zhou, L.F.; Cai, Y.F.; Liu, Y.; Liu, Z.W. Geochemistry and Geological Significance of the Permian Mudstone in the South Qilian Basin China. Bull. Miner. Petrol. Geochem. 2015, 34, 354–361. [Google Scholar]
- Li, F.L.; Xiao, F.; Meng, F.C.; Ren, Z.Y. Geochemical characteristics and implication for provenance of Upper Permian Linxi Formation clastic rocks in Solonker area, Inner Mongolia. J. Jilin Univ. (Earth Sci. Ed.) 2016, 46, 1769–1780. (In Chinese) [Google Scholar]
- Castorina, F.; Masi, U.; Padalino, G.; Palomba, M. Trace-element and Sr-Nd isotopic evidence for the origin of the Sardinian fluorite mineralization (Italy). Appl. Geochem. 2008, 23, 2906–2921. [Google Scholar] [CrossRef]
- Bau, M. Rare-earth element mobility during hydrothermal and metamorphic fluid-rock interaction and the significance of the oxidation state of europium. Chem. Geol. 1991, 93, 219–230. [Google Scholar] [CrossRef]
- Möller, P.; Holzbecher, E. Eu anomalies in hydrothermal fluids and minerals: A combined thermochemical and dynamic phenomenon. Freib. Forsch. 1998, 475, 73–84. [Google Scholar]
- Möller, P.; Bau, M.; Dulski, P.; Lüders, V. REE and Y fractionation in fluorite and their bearing on fluorite formation. In Proceedings of the Ninth Quadrennial IAGOD Symposium, Beijing, China, 12–18 August 1994; pp. 575–592. [Google Scholar]
- Simonetti, A.; Bell, K. Nd, Pb, and Sr systematics of fluorite at the Amba Donger carbonatite complex, India: Evidence for hydrothermal and crustal fluid mixing. Econ. Geol. 1995, 90, 2018–2027. [Google Scholar] [CrossRef] [Green Version]
- London, D.; Hervig, R.L.; Morgan, G.B. Melt-vapor solubilities and elemental partitioning in peraluminous granite–pegmatite systems: Experimental results with Macusani glass at 200 MPa. Contrib. Mineral. Petrol. 1988, 99, 360–373. [Google Scholar] [CrossRef]
- Zaraisky, G.P.; Korzhinskaya, V.; Kotova, N. Experimental studies of Ta2O5 and columbite-tantalite solubility in fluoride solutions from 300 to 550 °C and 50 to 100 MPa. Mineral. Petrol. 2010, 99, 287–300. [Google Scholar] [CrossRef]
- Keppler, H. Influence of fluorine on the enrichment of high field strength trace elements in granitic rocks. Contrib. Mineral. Petrol. 1993, 114, 479–488. [Google Scholar] [CrossRef]
- Badanina, E.V.; Syritso, L.F.; Volkova, E.V.; Thomas, R.; Trumbull, R.B. Composition of Li–F granite melt and its evolution during the formation of the ore-bearing Orlovka Massif in Eastern Transbaikalia. Petrology 2010, 18, 131–157. [Google Scholar] [CrossRef]
- Timofeev, A.; Williams-Jones, A.E. The Origin of Niobium and Tantalum Mineralization in the Nechalacho REE Deposit, NWT, Canada. Econ. Geol. 2015, 110, 1719–1735. [Google Scholar] [CrossRef]
- Timofeev, A.; Migdisov, A.A.; Williams-Jones, A.E. An experimental study of the solubility and speciation of tantalum in fluoride-bearing aqueous solutions at elevated temperature. Geochem. Cosmochim. Acta 2017, 197, 294–304. [Google Scholar] [CrossRef] [Green Version]
- Linnen, R.L.; Samson, I.M.; Williams-Jones, A.E. Geochemistry of the rare-earth element, Nb, Ta, Hf, and Zr deposits. Geology 2014, 13, 543–564. [Google Scholar]
- Bartels, A.; Vetere, F.; Holtz, F.; Behrens, H.; Linnen, R.L. Viscosity of flux-rich pegmatitic melts. Contrib. Mineral. Petrol. 2011, 162, 60–61. [Google Scholar] [CrossRef]
- Linnen, R.L.; Keppler, H. Columbite solubility in granitic melts; consequences for the enrichment and fractionation of Nb and Ta in the Earth’s crust. Contrib. Mineral. Petrol. 1997, 128, 213–227. [Google Scholar] [CrossRef]
- Linnen, R.L.; Cuney, M. Granite-related rare-element deposits and experimental constraints on Ta-Nb-W-Sn-Zr-Hf mineralization. In Rare Element Geochemistry and Mineral Deposits; Linnen, R.L., Samson, I.M., Eds.; Geological Association of Canada: St. John’s, NL, Canada, 2005; pp. 45–68. [Google Scholar]








| Type | Granite | Type I | Type II | Type III | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A-G | I-P | II-D | II-M | II-G | II-P | II-W | III P | ||||||||||||
| No. | 1 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 1 | 2 | 3 | 1 | 2 | 1 | 2 | 3 | 1 | 2 |
| Li | 133 | 4.82 | 4.02 | 4.17 | 17.4 | 16.7 | 18.4 | 12.3 | 11.3 | 0.04 | 0.03 | 0.03 | 0.63 | 0.78 | 0.27 | 0.25 | 0.24 | 0.16 | 0.26 |
| Be | 5.06 | 0.33 | 0.18 | 0.19 | 0.29 | 0.30 | 0.29 | 0.23 | 0.18 | 0.38 | 0.38 | 0.38 | 0.12 | 0.10 | 0.63 | 0.60 | 0.70 | 0.14 | 0.16 |
| Mg | 0.01 | 17.1 | 19.5 | 15.2 | 23.10 | 21.70 | 24.70 | 15.50 | 15.90 | 5.69 | 6.10 | 6.34 | 13.90 | 16.10 | 12.20 | 14.60 | 11.20 | 10.20 | 15.40 |
| Sc | 7.80 | 2.99 | 3.20 | 3.20 | 0.09 | 0.10 | 0.10 | 0.08 | 0.09 | 0.30 | 0.31 | 0.30 | 0.15 | 0.12 | 0.32 | 0.31 | 0.30 | 0.19 | 0.21 |
| Ti | <0.005 | 4.42 | 4.65 | 4.45 | 5.63 | 5.51 | 6.16 | 4.86 | 6.49 | 2.08 | 1.80 | 2.17 | 13.5 | 18.8 | 3.03 | 3.90 | 2.98 | 3.73 | 4.59 |
| V | <1.00 | 0.81 | 0.24 | 0.23 | 0.48 | 0.42 | 0.48 | 0.32 | 0.41 | 0.05 | 0.12 | 0.04 | 0.36 | 0.50 | 0.09 | 0.12 | 0.10 | 0.18 | 0.23 |
| Cr | 7.00 | 0.08 | 0.10 | 0.07 | 0.31 | 0.34 | 0.38 | 0.27 | 0.39 | 0.03 | 0.02 | 0.03 | 0.41 | 0.56 | 0.05 | 0.07 | 0.06 | 0.04 | 0.08 |
| Mn | 351 | 74.5 | 73.8 | 77.7 | 1.47 | 2.38 | 1.18 | 2.40 | 2.57 | 4.67 | 4.44 | 4.53 | 1.43 | 0.82 | 3.11 | 3.21 | 3.14 | 1.18 | 1.33 |
| Fe | 0.40 | 217 | 208 | 197 | 71.3 | 71.2 | 73.8 | 86.5 | 88.8 | 52.6 | 49.9 | 54.2 | 56.2 | 57.1 | 49.0 | 57.3 | 59.8 | 69.0 | 81.9 |
| Co | 0.20 | 0.02 | 0.03 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.14 | 0.05 |
| Ni | 0.60 | 0.32 | 0.30 | 0.25 | 0.06 | 0.04 | 0.02 | 0.11 | 0.13 | 0.11 | 0.05 | ||||||||
| Cu | 14.6 | 0.49 | 0.50 | 0.47 | 0.10 | 0.09 | 0.11 | 0.12 | 0.14 | 0.12 | 0.10 | 0.12 | 0.08 | 0.07 | 0.26 | 0.25 | 0.23 | 0.09 | 0.09 |
| Zn | 275 | 8.90 | 8.71 | 9.37 | 1.05 | 1.07 | 1.10 | 1.78 | 1.94 | 0.96 | 0.86 | 1.03 | 0.79 | 0.63 | 0.48 | 0.39 | 0.33 | 0.90 | 0.86 |
| Ga | 67.1 | 4.00 | 4.28 | 4.20 | 0.24 | 0.22 | 0.23 | 0.20 | 0.22 | 0.99 | 0.93 | 0.97 | 0.09 | 0.09 | 0.08 | 0.09 | 0.08 | 0.11 | 0.10 |
| Rb | 1570 | 20.1 | 18.6 | 18.4 | 0.87 | 1.05 | 0.80 | 0.54 | 0.57 | 0.13 | 0.12 | 0.14 | 0.20 | 0.24 | 0.25 | 0.34 | 0.28 | 1.06 | 0.71 |
| Sr | 6.80 | 139 | 136 | 131 | 129 | 128 | 131 | 127 | 129 | 140 | 141 | 141 | 152 | 159 | 105 | 109 | 107 | 76.6 | 80.5 |
| Y | 1.50 | 162.0 | 166 | 161 | 5.3 | 5.1 | 5.3 | 3.0 | 3.0 | 57.4 | 57.5 | 58.4 | 2.8 | 2.8 | 2.1 | 2.2 | 2.2 | 13.7 | 12.5 |
| Zr | 40.0 | 30.1 | 28.1 | 29.4 | 0.74 | 0.74 | 0.75 | 0.31 | 0.31 | 0.26 | 0.12 | 0.12 | 0.77 | 0.89 | 0.17 | 0.18 | 0.16 | 0.82 | 0.95 |
| Nb | 22.9 | 32.6 | 38.3 | 52.7 | 0.05 | 0.06 | 0.04 | 0.08 | 0.07 | 0.01 | 0.01 | 0.01 | 0.16 | 0.19 | 0.11 | 0.12 | 0.11 | 0.01 | 0.02 |
| Mo | 0.40 | 1.94 | 1.15 | 2.5 | 0.54 | 0.09 | 1.13 | 0.06 | 1.05 | 1.33 | 1.07 | 0.36 | 0.07 | 0.74 | 0.19 | 0.19 | 0.29 | 0.08 | 0.09 |
| Sn | 32.0 | 11.6 | 3.62 | 21.2 | 2.52 | 0.87 | 4.71 | 0.63 | 6.21 | 8.99 | 5.70 | 1.05 | 0.46 | 3.11 | 2.67 | 1.36 | 1.58 | 0.61 | 0.78 |
| Sb | 0.07 | 2.09 | 3.61 | 11.2 | 1.10 | 0.38 | 0.50 | 0.71 | 2.52 | 4.80 | 2.90 | 0.56 | 0.39 | 2.06 | 3.19 | 1.39 | 1.49 | 0.67 | 0.36 |
| Cs | 22.4 | 0.47 | 0.44 | 0.44 | 0.70 | 0.70 | 0.71 | 0.44 | 0.45 | 0.00 | 0.00 | 0.00 | 0.04 | 0.05 | 0.03 | 0.04 | 0.04 | 0.06 | 0.08 |
| Ba | 24.5 | 3.41 | 3.23 | 3.18 | 6.29 | 7.26 | 6.57 | 4.37 | 4.52 | 6.77 | 6.62 | 6.51 | 0.67 | 0.32 | 0.50 | 0.59 | 0.53 | 1.54 | 1.55 |
| La | 1.60 | 8.67 | 9.83 | 9.99 | 0.39 | 0.38 | 0.38 | 0.79 | 0.79 | 6.90 | 6.95 | 6.97 | 0.47 | 0.35 | 0.54 | 0.54 | 0.54 | 0.46 | 0.46 |
| Ce | 6.50 | 49.3 | 54.8 | 56.1 | 0.87 | 0.85 | 0.80 | 1.93 | 1.94 | 15.7 | 15.6 | 15.7 | 0.99 | 0.88 | 0.79 | 0.80 | 0.80 | 0.97 | 0.97 |
| Pr | 0.81 | 9.25 | 10.2 | 10.3 | 0.13 | 0.12 | 0.13 | 0.27 | 0.27 | 2.37 | 2.36 | 2.35 | 0.17 | 0.14 | 0.12 | 0.12 | 0.12 | 0.15 | 0.14 |
| Nd | 1.90 | 30.1 | 33 | 32.8 | 0.52 | 0.50 | 0.52 | 0.92 | 0.92 | 10.0 | 10.1 | 10.1 | 0.68 | 0.59 | 0.48 | 0.48 | 0.49 | 0.74 | 0.69 |
| Sm | 0.69 | 27.5 | 28.8 | 28.8 | 0.29 | 0.28 | 0.28 | 0.31 | 0.31 | 4.11 | 4.06 | 4.05 | 0.26 | 0.25 | 0.13 | 0.13 | 0.13 | 0.35 | 0.33 |
| Eu | 0.03 | 0.59 | 0.61 | 0.59 | 0.07 | 0.07 | 0.08 | 0.06 | 0.06 | 0.83 | 0.81 | 0.81 | 0.11 | 0.12 | 0.05 | 0.05 | 0.05 | 0.56 | 0.59 |
| Gd | 0.40 | 24.9 | 26 | 25.1 | 0.48 | 0.47 | 0.48 | 0.32 | 0.31 | 5.00 | 4.98 | 5.02 | 0.26 | 0.26 | 0.16 | 0.16 | 0.17 | 0.72 | 0.64 |
| Tb | 0.11 | 13 | 13.4 | 13 | 0.15 | 0.14 | 0.15 | 0.08 | 0.08 | 1.36 | 1.33 | 1.33 | 0.07 | 0.08 | 0.03 | 0.03 | 0.03 | 0.18 | 0.15 |
| Dy | 0.81 | 105 | 110 | 105 | 1.15 | 1.10 | 1.12 | 0.60 | 0.58 | 9.96 | 9.92 | 9.98 | 0.60 | 0.60 | 0.19 | 0.19 | 0.19 | 1.41 | 1.19 |
| Ho | 0.17 | 18.5 | 19.1 | 18.7 | 0.24 | 0.23 | 0.23 | 0.12 | 0.12 | 1.96 | 1.94 | 1.96 | 0.12 | 0.12 | 0.04 | 0.04 | 0.04 | 0.33 | 0.29 |
| Er | 0.64 | 70.5 | 72.6 | 70.5 | 0.74 | 0.71 | 0.73 | 0.36 | 0.36 | 6.27 | 6.14 | 6.13 | 0.43 | 0.44 | 0.12 | 0.12 | 0.12 | 1.12 | 0.95 |
| Tm | 0.19 | 19.8 | 20.7 | 19.8 | 0.13 | 0.13 | 0.13 | 0.07 | 0.07 | 1.18 | 1.19 | 1.19 | 0.10 | 0.10 | 0.02 | 0.02 | 0.02 | 0.21 | 0.18 |
| Yb | 2.20 | 191 | 196 | 192 | 0.83 | 0.79 | 0.82 | 0.49 | 0.49 | 8.67 | 8.57 | 8.54 | 0.89 | 0.95 | 0.11 | 0.11 | 0.11 | 1.61 | 1.44 |
| Lu | 0.38 | 25.4 | 26.3 | 25.4 | 0.12 | 0.12 | 0.12 | 0.08 | 0.08 | 1.25 | 1.25 | 1.24 | 0.15 | 0.16 | 0.02 | 0.02 | 0.02 | 0.28 | 0.26 |
| Hf | 12.1 | 7.22 | 6.91 | 7.1 | 0.03 | 0.02 | 0.02 | 0.01 | 0.01 | 0.03 | 0.03 | 0.03 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.03 | 0.03 |
| Ta | 29.0 | 7.5 | 7.46 | 9.25 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 |
| Pb | 94.8 | 30.7 | 31.8 | 29.5 | 0.51 | 0.52 | 0.54 | 1.17 | 1.18 | 0.29 | 0.28 | 0.27 | 0.14 | 0.07 | 0.43 | 0.41 | 0.40 | 0.26 | 0.32 |
| Th | 12.3 | 15.2 | 15.2 | 14.9 | 0.07 | 0.06 | 0.07 | 0.08 | 0.08 | 0.14 | 0.14 | 0.14 | 0.06 | 0.05 | 0.02 | 0.02 | 0.02 | 0.22 | 0.17 |
| U | 2.44 | 1.39 | 1.27 | 1.32 | 0.32 | 0.31 | 0.32 | 0.11 | 0.11 | 0.08 | 0.08 | 0.08 | 0.17 | 0.16 | 0.04 | 0.05 | 0.05 | 0.20 | 0.21 |
| Type | Type I | Type II | Type III | |||||
|---|---|---|---|---|---|---|---|---|
| I-P | II-D | II-M | II-G | II-P | II-W | III-P | ||
| No. | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 |
| Rba (ppm) | 20.1 | 0.87 | 0.54 | 0.12 | 0.20 | 0.25 | 0.34 | 1.06 |
| Sra (ppm) | 139 | 129 | 127 | 141 | 152 | 105 | 109 | 76.6 |
| 87Rb/86Sr | 0.4188 | 0.0195 | 0.0124 | 0.0025 | 0.0039 | 0.0069 | 0.0090 | 0.0401 |
| 87Sr/86Sr ± 2σ | 0.711708 ± 6 | 0.710237 ± 6 | 0.710212 ± 7 | 0.710181 ± 5 | 0.710175 ± 6 | 0.710392 ± 7 | 0.710398 ± 7 | 0.709099 ± 5 |
| (87Sr/86Sr)ib | 0.710861 | 0.710197 | 0.710187 | 0.710176 | 0.710168 | 0.710378 | 0.710380 | 0.709018 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Z.-P.; Jiang, S.-Y.; Su, H.-M.; Zhu, X.-Y.; Zou, T.; Cheng, X.-Y. Trace and Rare Earth Elements, and Sr Isotopic Compositions of Fluorite from the Shihuiyao Rare Metal Deposit, Inner Mongolia: Implication for Its Origin. Minerals 2020, 10, 882. https://doi.org/10.3390/min10100882
Duan Z-P, Jiang S-Y, Su H-M, Zhu X-Y, Zou T, Cheng X-Y. Trace and Rare Earth Elements, and Sr Isotopic Compositions of Fluorite from the Shihuiyao Rare Metal Deposit, Inner Mongolia: Implication for Its Origin. Minerals. 2020; 10(10):882. https://doi.org/10.3390/min10100882
Chicago/Turabian StyleDuan, Zhen-Peng, Shao-Yong Jiang, Hui-Min Su, Xin-You Zhu, Tao Zou, and Xi-Yin Cheng. 2020. "Trace and Rare Earth Elements, and Sr Isotopic Compositions of Fluorite from the Shihuiyao Rare Metal Deposit, Inner Mongolia: Implication for Its Origin" Minerals 10, no. 10: 882. https://doi.org/10.3390/min10100882