Abstract
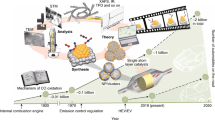
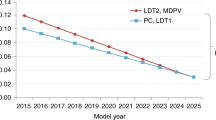
Advances in engine technologies are placing additional demands on emission control catalysts, which must now perform at lower temperatures, but at the same time be robust enough to survive harsh conditions encountered in engine exhaust. In this Review, we explore some of the materials concepts that could revolutionize the technology of emission control systems. These include single-atom catalysts, two-dimensional materials, three-dimensional architectures, core@shell nanoparticles derived via atomic layer deposition and via colloidal synthesis methods, and microporous oxides. While these materials provide enhanced performance, they will need to overcome many challenges before they can be deployed for treating exhaust from cars and trucks. We assess the state of the art for catalysing reactions related to emission control and also consider radical breakthroughs that could potentially completely transform this field.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Farrauto, R. J., Deeba, M. & Alerasool, S. Gasoline automobile catalysis and its historical journey to cleaner air. Nat. Catal. 2, 603–613 (2019).
Catalysis in motion. Nat. Catal. 2, 553–553 (2019).
Johnson, T. & Joshi, A. Review of vehicle engine efficiency and emissions. SAE Int. J. Engines 11, 1307–1330 (2018).
Bielaczyc, P. & Woodburn, J. Trends in automotive emission legislation: impact on LD engine development, fuels, lubricants and test methods: a global view, with a focus on WLTP and RDE regulations. Emission Control Sci. Technol. 5, 86–98 (2019).
Deutschmann, O. & Grunwaldt, J. D. Exhaust gas aftertreatment in mobile systems: status, challenges, and perspectives. Chem. Ing. Tech. 85, 595–617 (2013).
Twigg, M. V. Catalytic control of emissions from cars. Catal. Today 163, 33–41 (2011).
Twigg, M. V. & Phillips, P. R. Cleaning the air we breathe - controlling diesel particulate emissions from passenger cars. Platinum Metals Rev. 53, 27–34 (2009).
Zhang, R. D., Liu, N., Lei, Z. G. & Chen, B. H. Selective transformation of various nitrogen-containing exhaust gases toward N2 over zeolite catalysts. Chem. Rev. 116, 3658–3721 (2016).
Wang, A. & Olsson, L. The impact of automotive catalysis on the United Nations sustainable development goals. Nat. Catal. 2, 566–570 (2019).
Granger, P. & Parvulescu, V. I. Catalytic NOx abatement systems for mobile sources: from three-way to lean burn after-treatment technologies. Chem. Rev. 111, 3155–3207 (2011).
Lee, J., Theis, J. R. & Kyriakidou, E. A. Vehicle emissions trapping materials: successes, challenges, and the path forward. Appl. Catal. B 243, 397–414 (2019).
Raj, A. Methane emission control: a review of mobile and stationary source emissions abatement technologies for natural gas engines. Johnson Matthey Technol. Rev. 60, 228–235 (2016).
Beale, A. M. et al. Recent advances in automotive catalysis for NOx emission control by small-pore microporous materials. Chem. Soc. Rev. 44, 7371–7405 (2015).
Rappé, K. G. et al. Aftertreatment protocols for catalyst characterization and performance evaluation: low-temperature oxidation, storage, three-way, and NH3-SCR catalyst test protocols. Emission Control Sci. Technol. 5, 183–214 (2019).
De Rogatis, L. et al. Embedded phases: a way to active and stable catalysts. ChemSusChem 3, 24–42 (2010).
Li, G. & Tang, Z. Noble metal nanoparticle@metal oxide core/yolk-shell nanostructures as catalysts: recent progress and perspective. Nanoscale 6, 3995–4011 (2014).
Dai, Y. et al. A Sinter-resistant catalytic system based on platinum nanoparticles supported on TiO2 nanofibers and covered by porous silica. Angew. Chem. Int. Ed. 49, 8165–8168 (2010).
Peng, H. G. et al. Confined ultrathin Pd-Ce nanowires with outstanding moisture and SO2 tolerance in methane combustion. Angew. Chem. Int. Ed. 57, 8953–8957 (2018).
Arnal, P. M., Comotti, M. & Schüth, F. High-temperature-stable catalysts by hollow sphere encapsulation. Angew. Chem. Int. Ed. 45, 8224–8227 (2006).
Cargnello, M., Gorte, R. J. & Fornasiero, P. in Catalysis by Ceria and Related Materials 2nd edn (eds Trovarelli, A. & Fornasiero, P.) 361–396 (Imperial College Press, 2013).
Cargnello, M. et al. Exceptional activity for methane combustion over modular Pd@CeO2 subunits on functionalized Al2O3. Science 337, 713–717 (2012).
Adijanto, L. et al. Exceptional thermal stability of Pd@CeO2 core–shell catalyst nanostructures grafted onto an oxide surface. Nano Lett. 13, 2252–2257 (2013).
Li, L. et al. Hydrothermal stability of core–shell Pd@Ce0.5Zr0.5O2/Al2O3 catalyst for automobile three-way reaction. ACS Catal. 8, 3222–3231 (2018).
Seo, C. et al. Facile, one-pot synthesis of Pd@CeO2 core@shell nanoparticles in aqueous environment by controlled hydrolysis of metalloorganic cerium precursor. Mater. Lett. 206, 105–108 (2017).
Seo, C. Y. et al. Palladium redispersion at high temperature within the Pd@SiO2 core@shell structure. Catal. Commun. 108, 73–76 (2018).
Lu, J. L. et al. Coking- and sintering-resistant palladium catalysts achieved through atomic layer deposition. Science 335, 1205–1208 (2012).
Onn, T. M. et al. Modification of Pd/CeO2 catalyst by atomic layer deposition of ZrO2. Appl. Catal. B 197, 280–285 (2016).
Duan, H. et al. Pentacoordinated Al3+-stabilized active Pd structures on Al2O3-coated palladium catalysts for methane combustion. Angew. Chem. Int. Ed. 58, 12043–12048 (2019).
Mao, X. Y., Foucher, A., Stach, E. A. & Gorte, R. J. A study of support effects for CH4 and CO oxidation over Pd catalysts on ALD-modified Al2O3. Catal. Lett. 149, 905–915 (2019).
Getsoian, A. et al. Remarkable improvement in low temperature performance of model three-way catalysts through solution atomic layer deposition. Nat. Catal. 2, 614–622 (2019).
Morikawa, A. et al. A new concept in high performance ceria–zirconia oxygen storage capacity material with Al2O3 as a diffusion barrier. Appl. Catal. B 78, 210–221 (2008).
Chen, B. H.-Y. & Chang, H.-L. Development of low temperature three-way catalysts for future fuel efficient vehicles. Johnson Matthey Technol. Rev. 59, 64–67 (2015).
Toops, T. J., Parks, J., Choi, J.-S., Binder, A. & Kyriakidou, E. Low-Temperature Emission Control to Enable Fuel-Efficient Engine Commercialization (US Department of Energy, 2017).
Nishihata, Y. et al. Self-regeneration of a Pd-perovskite catalyst for automotive emissions control. Nature 418, 164–167 (2002).
Tanaka, H. et al. Design of the intelligent catalyst for Japan ULEV standard. Topics Catal. 30–31, 389–396 (2004).
Katz, M. B. et al. Self-regeneration of Pd-LaFeO3 catalysts: new insight from atomic-resolution electron microscopy. J. Am. Chem. Soc. 133, 18090–18093 (2011).
Onn, T. M. et al. Smart Pd catalyst with improved thermal stability supported on high-surface-area LaFeO3 prepared by atomic layer deposition. J. Am. Chem. Soc. 140, 4841–4848 (2018).
Neagu, D. et al. Demonstration of chemistry at a point through restructuring and catalytic activation at anchored nanoparticles. Nat. Commun. 8, 1855 (2017).
Song, Y. J. et al. Evolution of dendritic platinum nanosheets into ripening-resistant holey sheets. Nano Lett. 9, 1534–1539 (2009).
Cai, Y., Xu, J., Guo, Y. & Liu, J. Ultrathin, polycrystalline, two-dimensional Co3O4 for low-temperature CO oxidation. ACS Catal. 9, 2558–2567 (2019).
Yang, X. W. et al. Taming the stability of Pd active phases through a compartmentalizing strategy toward nanostructured catalyst supports. Nat. Commun. 10, 1611 (2019).
Yao, Y. et al. Carbothermal shock synthesis of high-entropy-alloy nanoparticles. Science 359, 1489–1494 (2018).
Xie, P. F. et al. Highly efficient decomposition of ammonia using high-entropy alloy catalysts. Nat. Commun. 10, 4011 (2019).
Loffler, T. et al. Discovery of a multinary noble metal-free oxygen reduction catalyst. Adv. Energy Mater. 8, 1802269 (2018).
Chen, H. et al. Entropy-stabilized metal oxide solid solutions as CO oxidation catalysts with high-temperature stability. J. Mater. Chem. A 6, 11129–11133 (2018).
Qiao, B. T. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Beniya, A. & Higashi, S. Towards dense single-atom catalysts for future automotive applications. Nat. Catal. 2, 590–602 (2019).
Liu, J. Catalysis by supported single metal atoms. ACS Catal. 7, 34–59 (2017).
Kropp, T. et al. Anionic single-atom catalysts for CO oxidation: support-independent activity at low temperatures. ACS Catal. 9, 1595–1604 (2019).
Lu, Y. B. et al. Identification of the active complex for CO oxidation over single-atom Ir-on-MgAl2O4 catalysts. Nat. Catal. 2, 149–156 (2019).
Goodman, E. D. et al. Catalyst deactivation via decomposition into single atoms and the role of metal loading. Nat. Catal. 2, 748–755 (2019).
Ganzler, A. M. et al. Tuning the structure of platinum particles on ceria insitu for enhancing the catalytic performance of exhaust gas catalysts. Angew. Chem. Int. Ed. 56, 13078–13082 (2017).
Datye, A. K. Dispersing nanoparticles into single atoms. Nat. Nanotechnol. 14, 817–818 (2019).
Peterson, E. J. et al. Low-temperature carbon monoxide oxidation catalysed by regenerable atomically dispersed palladium on alumina. Nat. Commun. 5, 4885 (2014).
Yao, Y. et al. High temperature shockwave stabilized single atoms. Nat. Nanotechnol. 14, 851–857 (2019).
Jones, J. et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 353, 150–154 (2016).
Kunwar, D. et al. Stabilizing high metal loadings of thermally stable platinum single atoms on an industrial catalyst support. ACS Catal. 9, 3978–3990 (2019).
Datye, A. & Wang, Y. Atom trapping: a novel approach to generate thermally stable and regenerable single-atom catalysts. Natl Sci. Rev. 5, 630–632 (2018).
Nie, L. et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 358, 1419–1423 (2017).
Pereira-Hernández, X. I. et al. Tuning Pt-CeO2 interactions by high-temperature vapor-phase synthesis for improved reducibility of lattice oxygen. Nat. Commun. 10, 1358 (2019).
Betz, B. Low Temperature CO Oxidation on Pt/CeO2 Containing Catalysts. PhD thesis, Ernst-Berl-Institut für Technische und Makromolekulare Chemie, Technical University of Darmstadt (2019).
Kim, C. H., Lee, H., Kang, C. Y. & Chung, J. W. For a new paradigm in aftertreatment: the almost zero concept for gasoline NOx and hydrocarbon emissions. In 27th Aachen Colloquium Automobile and Engine Technology (Aachener Kolloquium, 2018).
Bruix, A. et al. Maximum noble-metal efficiency in catalytic materials: atomically dispersed surface platinum. Angew. Chem. Int. Ed. 53, 10525–10530 (2014).
Betz, B., Muller, E., Hoyer, R. & Votsmeier, M. Low temperature CO/hydrocarbon oxidation in automobile exhaust using short pulse reductive activation of Pt/ceria. In 3rd Fundamentals and Applications of Cerium Dioxide in Catalysis (FACD, 2018).
Wei, S. J. et al. Direct observation of noble metal nanoparticles transforming to thermally stable single atoms. Nat. Nanotechnol. 13, 856–861 (2018).
Wang, H. et al. Surpassing the single-atom catalytic activity limit through paired Pt-O-Pt ensemble built from isolated Pt1 atoms. Nat. Commun. 10, 3808 (2019).
Jeong, H. et al. Highly durable metal ensemble catalysts with full dispersion for automotive applications beyond single-atom catalysts. Nat. Catal. 3, 368–375 (2020).
Leistner, K. & Olsson, L. Deactivation of Cu/SAPO-34 during low-temperature NH3-SCR. Appl. Catal. B 165, 192–199 (2015).
Wang, A. et al. Unraveling the mysterious failure of Cu/SAPO-34 selective catalytic reduction catalysts. Nat. Commun. 10, 1137 (2019).
Paolucci, C. et al. Dynamic multinuclear sites formed by mobilized copper ions in NOx selective catalytic reduction. Science 357, 898–903 (2017).
Paolucci, C. et al. Catalysis in a cage: condition-dependent speciation and dynamics of exchanged Cu cations in SSZ-13 zeolites. J. Am. Chem. Soc. 138, 6028–6048 (2016).
Gramigni, F., Selleri, T., Nova, I. & Tronconi, E. Catalyst systems for selective catalytic reduction + NOx trapping: from fundamental understanding of the standard SCR reaction to practical applications for lean exhaust after-treatment. React. Chem. Eng. 4, 1165–1178 (2019).
Jo, D. et al. Synthesis of high-silica LTA and UFI zeolites and NH3–SCR performance of their copper-exchanged form. ACS Catal. 6, 2443–2447 (2016).
Dusselier, M. & Davis, M. E. Small-pore zeolites: synthesis and catalysis. Chem. Rev. 118, 5265–5329 (2018).
Ji, Y., Bai, S. & Crocker, M. Al2O3-based passive NOx adsorbers for low temperature applications. Appl. Catal. B 170–171, 283–292 (2015).
Theis, J. R. An assessment of Pt and Pd model catalysts for low temperature NOx adsorption. Catal. Today 267, 93–109 (2016).
Chen, H.-Y. et al. Low temperature NO storage of zeolite supported Pd for low temperature diesel engine emission control. Catal. Lett. 146, 1706–1711 (2016).
Zheng, Y. et al. Low-temperature Pd/zeolite passive NOx adsorbers: structure, performance, and adsorption chemistry. J. Phys. Chem. C 121, 15793–15803 (2017).
Vu, A., Luo, J., Li, J. & Epling, W. S. Effects of CO on Pd/BEA passive NOx adsorbers. Catal. Lett. 147, 745–750 (2017).
Gu, Y., Zelinsky, R. P., Chen, Y. R. & Epling, W. S. Investigation of an irreversible NOx storage degradation mode on a Pd/BEA passive NOx adsorber. Appl. Catal. B 258, 118032 (2019).
Ballinger, T. H. & Manning, W. A., Lafyatis, D. S. Hydrocarbon Trap Technology for the Reduction of Cold-Start Hydrocarbon Emissions 970741 (SAE International, 1997).
Malamis, S. A., Harold, M. P. & Epling, W. S. Coupled NO and C3H6 trapping, release and conversion on Pd/BEA: evaluation of the lean hydrocarbon NOx trap. Ind. Eng. Chem. Res. 58, 22912–22923 (2019).
Haneda, M. & Hamada, H. Recent progress in catalytic NO decomposition. Comptes Rendus Chim. 19, 1254–1265 (2016).
Sun, Q. et al. A review on the catalytic decomposition of NO to N2 and O2: catalysts and processes. Catal. Sci. Technol. 8, 4563–4575 (2018).
Falsig, H. et al. Trends in catalytic NO decomposition over transition metal surfaces. Topics Catal. 45, 117–120 (2007).
Xu, W. et al. Development of MgCo2O4–BaCO3 composites as microwave catalysts for the highly effective direct decomposition of NO under excess O2 at a low temperature. Catal. Sci. Technol. 9, 4276–4285 (2019).
Monai, M., Montini, T., Gorte, R. J. & Fornasiero, P. Catalytic oxidation of methane: Pd and beyond. Eur. J. Inorg. Chem. 2018, 2884–2893 (2018).
Petrov, A. W. et al. Stable complete methane oxidation over palladium based zeolite catalysts. Nat. Commun. 9, 2545 (2018).
Danielis, M. et al. Outstanding methane oxidation performance of palladium-embedded ceria catalysts prepared by a one-step dry ball-milling method. Angew. Chem. Int. Ed. 57, 10212–10216 (2018).
Zhang, S. Y. et al. Dynamic structural evolution of supported palladium-ceria core-shell catalysts revealed by in situ electron microscopy. Nat. Commun. 6, 7778 (2015).
Riley, C. et al. Environmentally benign synthesis of a PGM-free catalyst for low temperature CO oxidation. Appl. Catal. B 264, 118547 (2020).
Glisenti, A. et al. Largely Cu-doped LaCo1−xCuxO3 perovskites for TWC: toward new PGM-free catalysts. Appl. Catal. B 180, 94–105 (2016).
Keav, S., Matam, S. K., Ferri, D. & Weidenkaff, A. Structured perovskite-based catalysts and their application as three-way catalytic converters—a review. Catalysts 4, 226–255 (2014).
Kang, S. B. et al. Coupled methane and NOx conversion on Pt+Pd/Al2O3 monolith: conversion enhancement through feed modulation and Mn0.5Fe2.5O4 spinel addition. Catal. Today https://doi.org/10.1016/j.cattod.2020.02.039 (2020).
Kim, C. H., Qi, G. S., Dahlberg, K. & Li, W. Strontium-doped perovskites rival platinum catalysts for treating NOx in simulated diesel exhaust. Science 327, 1624–1627 (2010).
Binder, A. J. et al. Low-temperature CO oxidation over a ternary oxide catalyst with high resistance to hydrocarbon inhibition. Angew. Chem. Int. Ed. 54, 13263–13267 (2015).
Yezerets, A. Understanding and modeling of aging is the key challenge for commercial vehicles. In Crosscut Lean Exhaust Emissions Reduction Simulations Workshop (CLEERS, 2019).
Lin, J., Wang, X. D. & Zhang, T. Recent progress in CO oxidation over Pt-group-metal catalysts at low temperatures. Chinese J. Catal. 37, 1805–1813 (2016).
Stonkus, O. A. et al. Thermally induced structural evolution of palladium-ceria catalysts. implication for co oxidation. ChemCatChem 11, 3505–3521 (2019).
Slavinskaya, E. M. et al. Metal–support interaction in Pd/CeO2 model catalysts for CO oxidation: from pulsed laser-ablated nanoparticles to highly active state of the catalyst. Catal. Sci. Technol. 6, 6650–6666 (2016).
Meng, L. et al. Synergetic effects of PdO species on CO oxidation over PdO–CeO2 catalysts. J. Phys. Chem. C 115, 19789–19796 (2011).
Hill, A. J. et al. Thermally induced restructuring of Pd@CeO2 and Pd@SiO2 nanoparticles as a strategy for enhancing low-temperature catalytic activity. ACS Catal. 10, 1731–1741 (2020).
Acknowledgements
The work at the University of New Mexico has been supported by NSF GOALI grant CBET-1707127 (catalyst ageing), the US Department of Energy (DOE), Office of Basic Energy Sciences (SC), Division of Chemical Sciences (grant DE-FG02-05ER15712) (catalyst synthesis) and the Air Force Office of Scientific Research (FA9550-18-1-0413) (computational modelling). The work at TU Darmstadt and Umicore has been supported by the German Federal Ministry for Economic Affairs and Energy (BMWi: 19U15014B) through the DEUFRAKO programme. We thank B. Betz for providing Fig. 7a, and we thank the following for helpful discussions: S. Oh, G. Qi and W. Li from GM Global R&D, C. H. Kim from Hyundai, C. Lambert from Ford, A. Yezerets from Cummins, K. Rappé from Pacific Northwest National Laboratory, T. Toops from Oak Ridge National Laboratory and N. Semagina from the University of Alberta.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Datye, A.K., Votsmeier, M. Opportunities and challenges in the development of advanced materials for emission control catalysts. Nat. Mater. 20, 1049–1059 (2021). https://doi.org/10.1038/s41563-020-00805-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-020-00805-3
This article is cited by
-
Water-assisted oxidative redispersion of Cu particles through formation of Cu hydroxide at room temperature
Nature Communications (2024)
-
Synthesis of core@shell catalysts guided by Tammann temperature
Nature Communications (2024)
-
Atomically dispersed materials: Ideal catalysts in atomic era
Nano Research (2024)
-
The effect of a gas atmosphere on the formation of colloidal platinum nanoparticles in liquid phase synthesis
Colloid and Polymer Science (2023)
-
Short Pulse Reductive Activation of Pt/ceria for the Low-Temperature CO Abatement in Vehicles Operated with the Synthetic Diesel Fuel OME
Topics in Catalysis (2023)