Abstract
Martensitic stainless steels are suitable for diverse structural applications but degrade when subjected to wear-prone activities in service. To enhance their service life, the densification of high Cr, martensitic, X190CrVMo20-4-1 tool steel powder on two different martensitic stainless steel substrates via supersolidus liquid-phase sinter (SLPS) cladding was investigated. The objective was to assess the influence of the difference in compositions of the martensitic stainless steels employed as substrates on the interfacial diffusion, microstructure, hardness and bonding strength of the steel-to-steel claddings. Computational thermodynamics and diffusion simulations were employed to supplement experimental findings. Owing to interdiffusion, a M7C3 carbide-free, banded region exists in the X190 adjacent to the interface with the width dictated by chemical potential gradient of carbon. The hardness of the substrate was lower near the interface region because of carbon enrichment, which promoted the presence of retained austenite. An interfacial strength of 798 MPa was achieved with fairly ductile X190 matrix near the cladding interface as the fracture surface was characterized by mixed fracture modes of dimple rupture and cleavage with localized quasi-cleavage features. Experimental observations and computational simulations are in agreement. The implications of the SLPS cladding technique are discussed in the context of tool development.
Similar content being viewed by others
1 Introduction
The combination of high strength and resistance to corrosion made martensitic stainless steels one of the choice materials for the development of structural components and parts of machinery for the food, chemical, mineral processing, oil and gas industries.[1,2,3,4] However, the degradation of these materials when subjected to wear- and corrosion-prone activities in service is inevitable. This necessitates the development of repair/remanufacturing processing routes. Different remanufacturing approaches have been investigated to repair worn parts and tools for reuse. These include sinter cladding, directed energy deposition processes, diffusion bonding and welding among others.[5,6,7,8,9,10] These approaches involve the overlay of material on the functional surface of the parent components or parts. The overlaid material can be of either similar or superior properties to that of the parent substrate material. Perhaps for cost-effectiveness, new parts or tools can be produced via these techniques, such that materials with premium properties are overlaid on the surface of an inexpensive substrate. It is mostly desired that a strong metallurgical bonding exists at the interface between the overlaid material and the substrate to avoid failure in service. By cladding, the opposing demands on certain regions of tools or parts can be satisfied. For example, the edge of a tool is mainly exposed to wear, while the remaining part of the tool should possess mainly good corrosion resistance and mechanical properties such as resistance against crack propagation. Wear-resistant materials are often comprised of hard reinforcement inclusions and phases, which makes the material more brittle and in case of carbides has a negative impact on the local corrosion resistance.
Blüm et al.[5] reported the sinter cladding of X245VCrMo9-4 tool steel powder on low carbon steel via a supersolidus liquid-phase sintering approach to achieve a wear-resistant surface on an inexpensive substrate tool suitable for mineral processing applications. A steel cladding with a strong metallurgical bonding owing to interdiffusion at the interface was achieved. In a similar study, a wear-resistant tool steel powder was sinter-clad onto three different low- and medium-carbon steels to investigate the interdiffusion profiles across the interfaces of the produced steel claddings.[11] The interdiffusion evaluation by both experimental and numerical approaches yielded similar profiles across the steel cladding interfaces. An up-hill diffusion of carbon from the substrate into the sinter-clad steel was noted to be influenced by a high content of nickel in one of the substrates owing to its high value of carbon activity. In another development, a multilayer sinter cladding of a tool steel and Hadfield steel was produced to achieve a tough and wear-resistant tool for industrial applications prone to dynamic loads and wear.[12] The interface between the steel cladding was characterized by an interdiffusion zone of about 40 µm width for the substitutional elements and the substrate grain boundary penetration diffusion. This promoted excellent metallurgical bonding as delamination did not occur at the interface of the steel cladding when subjected to the impact wear test.
Recently, the development of a bimetal consisting of high-chromium cast iron and low carbon steel via surface liquid-phase sintering, which is a similar process to supersolidus liquid-phase sinter cladding, was reported.[13] The bimetal was formed by sintering a mixture of low-carbon ferrochromium, graphite and ferromolybdenum powder preplaced on low carbon steel, and shear strength of 586 MPa was reported in the as-cast condition. The essence of the bimetal development is to synergize the mechanical properties of high-chromium cast iron with excellent wear resistance and high hardness and low carbon steel, which is ductile and tough, to achieve material suitable for both static and dynamic loads.[14,15,16] It is worth noting that the surface roughness of the steel substrate influences the bonding strength. Substrate surfaces, having numerous asperities with micro-relief grooves, exhibit poor wetting because of the surface tension of the liquid. This prevents the liquid from flowing into the micro-relief grooves, reduces surface contact area and lowers the bonding strength at the interface of a bimetal.[17] Since bonding occurs by diffusion during sinter cladding, the influence of surface roughness is considered minimal, provided there is adequate wetting of the substrate surface. Moreover, supersolidus liquid-phase sinter cladding is a novel approach in which a pre-alloyed metallic powder preplaced on a substrate material becomes simultaneously consolidated and bonded to the substrate. Densification is achieved by raising the temperature of the pre-alloyed powder above its solidus temperature to obtain a suitable volume of the liquid phase. To broaden the applicability of supersolidus liquid-phase sinter cladding, a high-chromium pre-alloyed tool steel powder consolidated and bonded on two martensitic stainless steels was considered in this study. This can be viewed as a repair/remanufacturing strategy for industrial tools to improve their wear resistance and extend their service life.
Thus, this study aims to investigate the supersolidus liquid-phase sinter cladding of a pre-alloyed high-chromium X190CrVMo20-4-1 tool steel on low-carbon cast GX20Cr14 and medium-carbon X46Cr13 martensitic stainless steel substrates. The X190CrVMo20-4-1 martensitic steel is a heat treatable tool steel widely used for the production of molds, dies and cutting tools for the food- and plastic-processing industries.[18,19] In a previous study, vacuum-sintered X190CrVMo20-4-1 steel produced via supersolidus liquid-phase sintering is characterized by the precipitation of chromium-vanadium-rich M7C3 carbides in a predominantly ferritic matrix.[20] Upon heat treatment, the matrix was transformed to martensite with a small amount of retained austenite. The dispersed M7C3 carbides had high hardness to effective elastic modulus ratio, which indicates their significance to the enhanced wear resistance of the X190CrVMo20-4-1 tool steel. However, this study considers the sinter cladding of this tool steel onto two martensitic stainless steels with high strength and excellent toughness. The interface microstructure, interdiffusion effects, hardness and phase constituents of the steel-to-steel claddings after heat treatment were examined. Experiments were supported with thermodynamic computations and diffusion simulation.
2 Materials and Methods
2.1 Materials
High-chromium X190CrVMo20-4-1 tool steel powder (X190) with a particle size of 45 ≤ d ≤ 62 µm was used in this study. The substrates upon which the steel powder was consolidated and bonded were a low-carbon cast GX20Cr14 (GX20) and a medium-carbon X46Cr13 (X46) martensitic stainless steels. Table I presents the elemental composition of all the steels employed in this study as obtained via a QSG750 spark optical emission spectrometer.
2.2 Computational Thermodynamics and Diffusion Simulation
Before sinter-cladding experiments, computational thermodynamics was employed to evaluate the solidus temperatures and the liquid phase evolution of the steels to be processed via SLPS under equilibrium conditions. A ThermoCalc Software version 2020.1 package with the TCFE9 database in conjunction with the elemental compositions presented in Table I was used for the computation. The evaluation assisted in the choice of the densification temperature for the high-chromium tool steel powder and the maximum sintering temperature to avoid melting of the steel substrates. Moreover, based on the elemental composition of the steel, diffusion simulations were also made to predict the elemental interdiffusion across the interface of the steel claddings. A coupled thermodynamic-kinetic modeling software, DICTRA, using TCFE9 and MOBFE5 databases with Gibbs Energy System version 5, was employed for the calculations. A domain length of 5 mm across the interface was considered, which was divided into two regions by the Heaviside step function, hs(x). The whole domain length was double geometrically discretized to 200 grid points with a higher point density in each region towards their interface. The simulation was conducted for the temperature and the duration at which the processing of the steel-to-steel claddings was carried out. At this temperature, austenite is considered as the continuous matrix phase in both the sintered X190 steel and the substrate steels in which diffusion is assumed to occur. However, other phases (MC carbide and liquid) present in the densified X190 steel at this temperature are considered as dispersed phases, which attenuate the interdiffusion path.[21,22] Hence, the influence of the carbide and the liquid phases on the elemental diffusion across the interface was taken into consideration by the implementation of a dispersed phase model with labyrinth factor, λ(fm), taken as in Eq. [1], in the DICTRA simulation. The labyrinth factor takes care of the impeding effect of the considered dispersed phases on the diffusion coefficient matrix (Eq. [2]), especially for long-range diffusion in a multi-component system such as employed in this study.[23]
where λ(fm) is the labyrinth factor, which is a function of the volume fraction of the continuous matrix phase, fm, \( D_{kjeff}^{n} \) is the effective diffusion coefficient matrix, and \( D_{kj}^{n} \) is the diffusion coefficient matrix.
2.3 Sinter-Cladding Process
A steel substrate (5 × 5 × 60 mm3) was placed in an alumina crucible, and X190 steel powder (51.3 g) was placed on top of the substrate at tap density. The crucible was placed in a model 7/75 tubular vacuum furnace for the sinter-cladding process. The furnace temperature was raised at a heating rate of 10 K/min to the densification temperature. The densification temperature was held for 30 minutes while the furnace chamber was maintained under a vacuum condition. Thereafter, the sinter-clad steel was allowed to cool in the furnace to room temperature. Before the setting up, the substrate steels were cleaned with ethanol to prevent surface contamination.
2.4 Heat Treatment
Specimens (5 mm length) were cut from the as-sinter-clad samples and subjected to austenitization at 1150 °C for 30 minutes before quenching in oil. Thereafter, the specimens were subjected to tempering at 200 °C for 2 hours.
2.5 Characterization
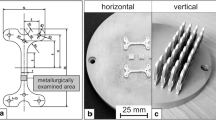
Specimens were prepared for microstructural examination by embedding them in a conductive resin and then grinding and polishing them to a mirror finish using a 1-µm diamond suspension solution. After polishing, samples were etched using V2A etchant [a mixture of 100 mL distilled water (H2O), 100 mL hydrochloric acid (HCl), 100 mL nitric acid (HNO3) and 0.3 mL Vogels inhibitor][24] to reveal the microstructure of the steels. The microstructural characterization was made using a Tescan Vega3 SBH scanning electron microscope (SEM) operated at an accelerating voltage of 20 kV coupled with energy-dispersive X-ray (EDS) system. A Bruker D2 Phaser X-ray diffractometer operated at a voltage of 30 kV and 20 mA current to generate a Cu K (α) radiation was used to record the patterns of the steels in quenched and tempered condition. XRD patterns were acquired at an angular step size of 0.02 deg and a 3 seconds counting time per data point over a 2θ range of 40 to 90 deg. Phase identification was achieved by matching the reflections in the measured diffractograms with phase patterns in the database of the International Centre for Diffraction Data (ICDD). Diffractograms were acquired for the bulk X190 and substrates at a plane that is 3 mm distance from the interface and also at a near-interface plane in the substrates. Experimental pattern data were analyzed based on the Rietveld method using a pattern-fitting procedure for phase quantification.[25,26] The hardness of the specimens was assessed using an ATM Carat 930 hardness tester. For 10-s dwell time, macro-hardness of the steel samples was assessed with an applied load of 98.1 N (10 kg), and the hardness profile across the interface of the steel claddings was measured with a load of 4.9 N (0.5 kg) applied. Shear tests were conducted on a Zwick/Roell Z050 universal testing machine to determine the interfacial strength of the steel claddings as schematically illustrated in Figure 1. A 2 N preload and a crosshead speed of 5 mm/min were applied during the tests, and the shear strength is determined using Eq. [3].
where τ is the shear strength (MPa), Fmax is the maximum force applied (N), and A is the cross-sectional area (mm2).
3 Results and Discussion
3.1 Computational Thermodynamics
The evolution of the liquid phase in the tool steel during sintering is essential to achieve its densification with minimal or no porosity and excellent bonding to the steel substrates. By computational thermodynamic evaluation using the elemental composition, the volume of liquid phase expected in both the tool steel powder and steel substrates as a function of temperature is depicted in Figure 2(a). The solidus temperatures of the steel substrates are higher (> 1400 °C) than that of the X190 tool steel (1249 °C). In addition, the steel substrates are expected to remain solid, even when the tool steel has reached its 100 vol pct liquid phase. Based on a previous study, the vacuum sintering temperature of 1280 °C was adopted, as it resulted in a 99.9 ± 0.01 pct densification of the X190 steel powder.[20] Since there was no liquid phase fraction expected in the substrates, the bonding between the sintered X190 steel powder and the substrates is expected to be dominated by elemental diffusion. However, other bonding phenomena, such as crystallographic allomerism and the same atomistic bonding and solubility, are possible to interplay.
Upon slow cooling in the furnace, the solidification of the liquid phase in the X190 steel occurs. Figure 2(b) shows the Scheil solidification curve for the liquid fraction in the X190 steel as the temperature decreases during cooling, assuming carbon as a fast diffusing element under global minimization condition. At 1280 °C, the phases predicted to be present in the X190 are liquid, austenite and MC carbides. As cooling occurs, the nucleation and growth of M7C3 carbides are expected to begin at 1267 °C. The solidification continues with a decrease in temperature, and 99 wt pct solid is expected at 1215 °C.
3.2 Diffusion Simulation
The interfacial bonding between the sintered tool steel and the steel substrates is dependent on the elemental interdiffusion of their adjacent grains. The elemental interdiffusion across a diffusion couple occurs from the region with a higher concentration than that with a lower concentration, according to Fick’s second law.[27] In addition, the driving force that governs the net diffusion direction relies on the gradient of the chemical potential of the diffusing elements as given by Eq. [4], and the chemical potential of each element is dependent on the thermodynamic activity of the element at a predetermined temperature of the steel as shown in Eq. [5].[28]
where F is the driving force, x is the diffusion distance, µ is the chemical potential of the element, µ0 is the chemical potential of the element at standard condition, R is the gas constant, T is the temperature, and a is the thermodynamic activity of the element.
However, in multi-component systems such as the steels considered in this study, chemical reactions in the form of carbide precipitations are expected, especially in X190. This indicates that a fraction of the entire molar concentration of each element is actively involved in the interdiffusion across the interface of the steel claddings. This influences the chemical activity of each element in individual steel. Based on the elemental composition of these steels (Table I), the interdiffusion of some selected elements, C, Cr, V and Mo, across the interface of the steel claddings was evaluated. These elements were considered for evaluation owing to their substantial contents in the steels. Table II presents the chemical potential of the elements in the steels employed in this study. As the chemical potential is the driving force, the atoms of each element diffuse towards the interface from the steel with higher chemical potential into the steel with lower potential. The chemical potentials of carbon, vanadium and molybdenum in the sintered X190 steel are higher than that of the substrates. Hence, the atoms of these elements are expected to diffuse from X190 steel across the interface into the substrates. Despite a higher content of chromium in the X190 steel than that of the substrates (Table I), the chemical potential of Cr in X190 is only higher than that in the X46 substrate. This indicates that the diffusion of chromium is expected from the GX20 substrate into the sintered X190 steel, which is an uphill diffusion. According to the Darken equation, there is a chance for the concentration gradient of an element in a multi-component system to cause an uphill diffusion of another element in a diffusion couple.[22]
Figure 3 shows the interdiffusion profiles of the selected elements across the interface. The trend of the elemental profiles indicates that the interdiffusion across the interface is chemical potential-driven. The higher the difference in the chemical potential in the adjacent steels is, the higher the concentration gradient towards the interface. The interdiffusion region, where the elements actively diffuse across the interface, is noticeable as the distance from where the local elemental concentrations are maintained in the adjacent steels. Of all the elements, carbon has the highest calculated diffusion length ranging between 1500 to 2000 µm across the interface (Figure 3(a)). The carbon diffusion length of about 500 µm from the interface is calculated in the X190, while the distance is 1000 µm in X46 and 1500 µm in GX20. Carbon has a long-range diffusion potential as it diffuses and occupies interstitial sites in the steel matrix. However, the diffusion length across the interface for the substitutional elements (Cr, V and Mo) ranges between 30 and 40 µm (Figures 3(b) through (d)). A gradual decrease in the global content of the substitutional elements in the X190 is noticeable at about 10 µm from the interface. The concentration of the substitutional elements decreases gradually to the global concentration of these elements in the steel substrate at a distance of 20 to 30 µm from the interface. Besides the chemical potential gradient, the diffusion of the substitutional elements relies on the availability of vacant sites to be occupied, which is responsible for their short-range diffusion. The uphill diffusion of Cr from the GX20 steel substrate into the sintered X190 steel is observed in the profile with a little upward shift in the X190 immediately after the interface. This indicates that the X190 region near the interface is slightly enriched with Cr from the GX20 steel. Thus, the region of the GX20 adjacent to the interface is highly enriched by C, V and Mo diffusion from the X190 tool steel owing to a high chemical potential difference. With the diffusion of carbon and other substitutional elements, microstructural modification of the steels in the near-interface region is anticipated.
Calculated elemental diffusion profiles across the interface of sintered X190 and the X46 and GX20 steel substrates at 1280 °C with a dwell time of 30 min: (a) C; (b) Cr; (c) V; (d) Mo. Red dotted lines indicate the interface position between the steels and black dotted lines indicates the global element contents in the steels (Color figure online)
Based on the interdiffusion simulation, a steep concentration gradient for C is observed in the X190 toward the interface with the GX20 substrate, with the global C content in X190 (1.82 wt pct) lowered to 1.50 wt pct. However, a gentle gradient is observed toward the interface with X46, and the global C concentration in X190 decreases slightly to 1.76 wt pct (Figure 3(a)). The carbon concentration gradient is higher in GX20 than in X46 from the interface into the substrates. From the interface, the concentration gradients for Cr and Mo are higher in X46 steel than in GX20 steel (Figures 3(b), (d)). The difference in their concentration gradient is dependent on the difference in the local concentration level of each element in the steel substrates, as the concentration gradient of V for both substrates is similar (Figure 3(c)).
3.3 Microstructure of the steel claddings
The microstructure of the steel claddings was examined after oil quenching at 1150 °C and tempering at 200 °C for 2 hours. For effective wear resistance of the tool steel, the carbide precipitates must be dispersed uniformly in a martensitic matrix. Figure 4 depicts the SE-SEM micrographs of the sintered X190 tool steel and the substrates at a distance of 3 mm away from their interface. The microstructure of the sintered X190 steel powder is characterized by the precipitation of the M7C3 carbides and presumably fine MC carbides in a martensitic matrix as seen in Figures 4(a) and (b). The steel substrates X46 (Figure 4(c)) and GX20 (Figure 4(d)) exhibit a martensitic microstructure after heat treatment. However, a little volume of retained austenite is expected to be present in the matrices of both the sintered X190 and the substrate steel after the conventional oil quenching owing to the chemistry of the austenitic matrix at the quench-hardening temperature.[20]
Table III presents the prior-austenite grain size of the steel after heat treatment determined using mean linear intercept method and the grain size number calculated using Eq. [6] according to ASTM E112-12. The sintered X190 steel possessed finer grains (51 µm) compared to those of the substrate (GX20, 174 µm; X46, 192 µm). The grain size numbers of the substrates were relatively similar (< 2) while that of the sintered X190 steel is higher (5.3).
where in Eq. [6] G is the grain size number and l is the mean intercept length of the steel grain in mm.
By image binarization of five (5) SE-SEM micrographs of the sintered X190 steel microstructure, the volume percentages of the carbides after heat treatment were measured and presented in Table IV. At a low magnification such as Figure 4(a), the percentage of the M7C3 carbides measured in the microstructure is 16.67 ± 0.76 pct, while the remaining 83.33 pct is the matrix and the MC carbides. However, it is impossible to accurately measure the volume percent of MC carbides in the microstructure at this low magnification. Since the MC carbides are predominantly observed within the X190 steel grains, the percentage of the MC carbides is measured over a high magnification micrograph of these predominantly clustered regions to give 3.03 ± 0.32 pct and the remaining is the matrix. Since at low magnification the volume percent of the MC carbide and the matrix is 83.33 pct, then the volume percent of the MC carbides is calculated to be 2.5 pct with an assumption that there is a uniform dispersion of the MC carbides globally in the steel matrix.
Moreover, the measured size distribution of the MC carbides in the sintered X190 steel is presented in Figure 5. The carbide size distribution is right-skewed, and precipitates with size ≤ 1 µm have the highest frequency. The mean carbide size is 0.90 ± 0.03 µm, and precipitate size ranges from 0.19 to 4.03 µm. However, the frequency of the MC carbide with size > 2 µm is small.
Figure 6 depicts the microstructure of the sintered X190 steel in the quenched and tempered condition with locations for EDS point analysis on the fine MC carbide and the matrix and area analysis on the coarse M7C3 carbide. The semi-quantitative analyses of the elemental composition of the carbides and the matrix of the sintered X190 steel at locations in Figure 6 are presented in Table V. The synthesized MC carbides have a near-stoichiometric composition of about 50 at. pct carbon as expected while the remaining 50 at. pct is a combination of metallic elements, especially Fe, Cr and V. Moreover, the M7C3 carbides have mainly 35 at. pct carbon, 36 at. pct Cr, 17 at. pct Fe and 11 at. pct V. Thus, the M7C3 carbides are Cr-richer and V-richer than the MC carbides in the vacuum-sintered X190 steel. As for the steel matrix, carbon was left unquantified as its value was unreasonable; however, the matrix possesses 15 at. pct Cr, which is expected to significantly enhance its resistance against corrosion attack in service.
After oil quenching and tempering, the presence of retained austenite is evident in the steel as observed in the optical micrographs in Figure 7. The retained austenite is observed to have a bright, blocky morphology within the martensitic matrix and the region around carbides in the X190 steel (Figure 7(a)). During the austenitisation at 1150 °C, the regions around the carbides in X190 steel are enriched by carbon and other elements owing to the diffusion from the carbides. This promotes the stabilization of the austenite phase around the region after the conventional oil quenching.[29] In the steel substrates (Figures 7(b) and (c)), the retained austenite is observed among the plate and lath martensite within their prior-austenite grains. At the interface region of the steel claddings, the presence of retained austenite is more apparent as evident from Figures 7(d) and (e). The interface region is enriched by carbon owing to elemental interdiffusion between the sintered X190 and the substrate, which is a major stabilizer of austenite phase and promotes the presence of retained austenite after heat treatment. The presence of retained austenite extends from the sintered X190 across the interface into the steel substrate, as evident by the region around the grain boundary, marking the transition between the cladding and the steel substrate.
Since the formation of martensite from the austenite state of the steels when quenched is a diffusionless transformation process,[30] the elemental composition of the retained austenite is considered to be the same as the elemental composition of the austenite phase of the steel prior to quenching from 1150 °C. Table VI shows the thermodynamically calculated elemental composition of the different phases present in the sintered X190 steel as the austenitisation temperature of 1150 °C. The formation of the carbide precipitates (MC and M7C3) has consumed the carbon content of the steel, such that the austenite matrix phase at the austenitisation temperature of 1150 °C is expected to possess 0.39 wt pct carbon. Hence, the retained austenite in the sintered X190 is expected to have a similar composition as the γ-Fe phase in Table VI. Moreover, the steel substrates remained austenitic without carbide precipitates at 1150 °C; thus, the composition of the retained austenite in these steel substrates is considered the same as their elemental compositions in Table I.
Figure 8 shows the SE-SEM micrograph of the interface region of the steel claddings. The claddings exhibit a desirable metallurgical bonding at their interfaces, attributed to an effective interdiffusion as predicted in Figure 3. The matrices of both the sintered X190 and the substrates at the interface region remained martensitic as observed at a distance of 3 mm away (Figure 4). A region with only fine MC carbides and no M7C3 carbides is observed in the sintered X190 steel adjacent to the interface of the steel claddings. Within this region, grain boundaries are established between the sintered X190 steel and the steel substrates, as observed in Figures 8(b) and (d). Having the grain boundaries linking the steels in this region with fine carbide precipitates is considered beneficial, as susceptibility to failure at the interface owing to coarser M7C3 carbide is lowered when employed in service. This banded region adjacent to the interface is observed to be wider for the X190/GX20 steel cladding (52 ± 4 µm) than that of the X190/X46 steel cladding (23 ± 2 µm). This is attributed mainly to the large chemical potential difference of carbon in the X190 and GX20 steels (Table II). This leads to a high concentration gradient of carbon as diffusion occurs towards the GX20 steel substrate. As predicted by the carbon diffusion simulation across the interface of the steel claddings (Figure 3(a)), the concentration of C in the X190 steel towards the interface with the GX20 steel indicates a steeper gradient than that observed toward the X46 steel. Thus, the intense carbon diffusion gradient from the sintered X190 steel across the interface into the medium carbon X46 and low carbon GX20 steel substrates promotes the formation of the zone free of coarse M7C3 carbide in X190 adjacent the interface.
3.4 EDS Line Scan Across the Interface of the Steel Cladding
Figure 9 shows the SE-SEM micrographs of the interface region between the sintered X190 steel and steel substrates (X46 and GX20) with positions of EDS line scans. Generally, a low to near-zero concentration gradient was observed across the interface of the steel claddings for the majority of the elements assessed by EDS. Moreover, a noticeable concentration gradient is observed for Cr and V profiles. This is attributed to a relatively large compositional difference of these elements in the sintered X190 steel and the steel substrates compared to other substitutional elements. The corresponding Cr and V concentration profiles across the interface of the steel claddings are presented. The formation of the M7C3 carbides is obvious within the sintered X190 steel, and, by contrast, a region without the M7C3 carbide precipitates is seen between the sintered X190 and steel substrate. This region is more apparent for the X190/GX20 steel cladding as earlier observed in Figure 8. Besides carbon not being quantified, it is observed that the M7C3 carbides are enriched by all the substitutional elements, especially Cr and V, as shown in Figures 9(b) and (d) and seen in Table V. Within the sintered X190 steel, the M7C3 carbides possess about 55 wt pct Cr and 17 wt pct V, while the matrix possesses about 15 wt pct Cr and 1.5 wt pct V, as seen in the EDS profile in Figures 9(b) and (d).
SE-SEM images of the unetched interface region between the sintered X190 steel and the substrates (a) X46 steel and (c) GX20 steel, after oil quenching at 1150 °C and tempering at 200 °C for 2 h. (b) EDS line scan profile of Cr and V across arrow AB in (a); (d) EDS line scan profile of Cr and V across arrow CD in (c)
Owing to the chemical reaction leading to the formation of Cr-rich carbides in X190 steel, as predicted in Figure 2(b), the Cr content of the steel matrix is reduced to a range between 12 and 15 wt pct as determined by EDS without carbon quantification.[19] This is considered responsible for the slightly lower chemical potential of Cr in the X190 when compared to that of the GX20 steel (Table II). Hence, an uphill diffusion of Cr from the GX20 steel substrate towards the sintered X190 steel is favored, as observed in Figure 3(b). The diffusion length across the interface for these substitutional elements is observed to be between 30 and 40 µm, as observed in the insets in Figures 9(b) and (d). The experimentally measured diffusion length for these substitutional elements is in agreement with the interdiffusion distance across the steel claddings predicted by diffusion simulation using DICTRA (Figures 3(b) and (c)). As seen in Figures 9(b) and (d), the Cr concentration gradient across the interface of X190/X46 is steeper when compared to that across the X190/GX20 steel cladding. This is attributed to the difference in the global concentration of Cr in the steel substrates [X46 (12.7 wt pct) and GX20 (14.17 wt pct)], as seen in Table I. However, the steepness of the V diffusion gradient across the interface is similar for both steel claddings. The observed concentration gradients of these elements are in agreement with those predicted via simulation, as shown in Figures 3(b) and (c).
Though the carbon profile across the interface of the steel claddings cannot be accurately measured by EDS line scans, the C diffusion simulation profile (Figure 3(a)) is considered reliable. Previous work has established a good agreement between the elemental profile of steels determined by both simulation and experiment.[11] In addition, the predictions of the diffusion length and concentration gradients of substitutional elements by the diffusion simulation are in agreement with the EDS results. During the sinter cladding, the liquid phase is required for the densification of the X190 steel powder, and at the interface, rapid diffusion of C into the substrate is promoted. The carbon diffusion from X190 steel into the adjacent region of the steel substrates makes it lean in carbon, and this raises the solidus temperature of the X190 near the interface.[11] Figure 10 shows the volume of the liquid phase across the interface of the steel claddings. The volume of the X190 steel liquid phase is observed to decrease towards the interface with the substrate being solid at 1280 °C. The carbon diffusion is considered responsible for the decrease in the X190 liquid phase volume towards the interface. However, the liquid volume in the sintered X190 steel near the interface of the steel claddings is considered sufficient, as the interface is pore-free (Figure 8). The loss of carbon from the X190 to the substrate is responsible for the necessity of superheating the tool steel. The liquid phase volume may be considered too high in SLPS to reach an optimum microstructure, free of eutectic, coarse carbides; however, there is a need for a high sintering temperature to compensate the carbon loss and obtain fully dense steel.
As the elemental interdiffusion occurs, the sintered X190 steel region adjacent to the interface with the substrates becomes lean in carbon. This lowers the chance for the formation of coarse M7C3 carbides in the region. This banded region with fine near-spherical MC carbides is evident in Figures 8(b) and (d). As determined via simulation and EDS line scans, the diffusion lengths of the substitutional elements (Cr, V, Mo) across the interface of the steel claddings are similar, ranging between 30 and 40 µm. Based on the diffusion simulation, the diffusion distance is asymmetrical in relation to the steel cladding interface. Thus, carbon diffusion is considered to be mainly responsible for the larger banded region observed in the X190 steel adjacent to the interface with GX20 steel (Figure 8(c)) compared to that with X46 steel (Figure 8(a)). This is corroborated by the lowered X190 liquid volume at the interface with GX20 steel compared to that with X46 steel (Figure 7).
3.5 Phase Constituents
After subjecting the steel claddings to heat treatment by oil quenching at 1150 °C and tempering at 200 °C for 2 hours, the diffractograms of the steels were recorded. The phase constituents were determined by matching reflections with the ICDD database. Experimental data were fitted by the Rietveld method to determine the phase compositions. Figure 11 shows the fitted patterns overlapped on the experimental patterns of the heat-treated steels forming the claddings. In all the diffractograms, reflections of martensite (α′-Fe) and retained austenite (γ-Fe) are present with the chromium-rich carbide (M7C3) phase additionally identified in the XRD pattern of X190 steel. In the bulk X190, X46 and GX20 steels at 3 mm away from the interface, martensite is identified as the dominant phase with 100 pct reflection at the α′-Fe (110) plane. Moreover, the reflections of γ-Fe (111) and α′-Fe (110) in the XRD patterns obtained from a plane in X46 and GX 20 at the interface region with the X190 are of similar intensities.
Table VII shows the volume percent of the phases present in the steels with their corresponding weighted-pattern residuals. In all, the fitted patterns by the Rietveld method are similar to the experimental diffractograms. The fit parameters indicated a close agreement with the experimental pattern with the goodness of fit χ2 ≤ 0.1 and the weighted-pattern residual Rwp < 10 pct. The Rwp is a measure of the convergence of the refinement, and it should have a value < 15 pct.[31] In Table VII, the predominantly measured phase in all the steels after heat treatment at 3 mm away from the cladding interface is the martensite. This is in agreement with the microstructural observations in Figure 4 and their strong and highest reflections at the (110) plane in Figure 11. However, the ratio of martensite to the retained austenite at the interface region of the GX20 substrate is about 0.6:0.4, while that of the X46 substrate is vice versa. By comparing the optical micrograph of the interface regions (Figures 6(d) and (e)), the retained austenite is concentrated within the interface region extending from the narrow coarse M7C3 carbide zone into the X46 substrate. However, the M7C3 carbide free zone at the X190/GX20 interface is wider, and the retained austenite is present within this region. The increase in the retained austenite at the interface region in the X46 and GX20 substrates is due to the elemental interdiffusion from the sintered X190 across the interface into the substrates (Figure 7). Thus, the near interface region of the substrate is carbon enriched without coarse M7C3 carbides formed, which strongly influences the stabilization of austenite after oil quenching at 1150 °C. The matrix of the steel claddings is expected to be martensitic to make them wear-resistant. Thus, the matrices of both the sintered X190 steel and the substrates became martensitic upon quenching at 1150 °C, and the low tempering temperature of 200 °C helped to relieve the internal stress in the matrix. However, it may be impossible to achieve a fully martensitic matrix by conventional oil quenching, as undercooling of about 200 °C from the martensite start temperature, Ms, to martensite finish temperature, Mf, cannot be achieved. This makes the temperature-dependent martensitic transformation to remain incomplete, and the untransformed portion of the steel matrix remained as retained austenite.[32,33] Hence, the reflections of the retained austenite phase become inevitable in the XRD patterns of the quenched and tempered steel claddings.
The quantification of Cr-rich M7C3 carbides from the fitted pattern of the heat-treated X190 steel via the Rietveld simulation (23.3 ± 1.5 vol pct) is slightly higher than the experimentally measured value of 16.67 ± 0.76 pct via image processing (Table IV). The exaggeration may have been due to the over-fitting of the XRD experimental data for the X190 during the Rietveld simulation. By computational thermodynamics, the quantity of M7C3 carbides present in the X190 steel when quenched at 1150 °C is 17.78 vol pct, as shown in Table IV. This calculated value is similar to the obtained value via image processing. In addition, the MC carbides identified in the microstructure (Figure 4) are calculated as 0.73 vol pct of the X190 steel composition (Table VIII). Hence, the non-identification of this phase in the XRD pattern of the X190 is due to its small volume fraction, as measured to be 3 vol pct on a highly magnified micrograph (Table IV). Since the measured volume fraction of the M7C3 carbides is similar to the thermodynamically calculated value, the calculated global volume fraction of the MC carbide in Table IV is assumed to be correct. In addition, the reflections of MC carbides may be inseparable from that of γ-Fe, as both phases possess a similar crystallographic structure and lattice parameters. From the calculated data in Table VIII and the phase composition of X190 in Table VI, it can be deduced that about three-quarters of the steel matrix is transformed to martensite, while a quarter remained untransformed as austenite after the oil quenching.
3.6 Hardness
As the performance of tool steels in combating wear is related to their hardness, the macro-hardness of the steel claddings in the as-sintered and heat-treated conditions was assessed and is presented in Table IX. In the as-sintered condition, the X190 tool steel had the least hardness of 265 ± 3 HV10. However, upon oil quenching at 1150 °C, the sintered X190 steel had the highest hardness of 749 ± 5 HV10. The increased hardness is primarily due to the transformation of most of the steel matrix (three quarters) to martensite upon quenching, as observed in the microstructure (Figure 4) and the phase composition in Table VII.
For tool steel, the optimum quench-hardening temperature is dependent on the carbon content of the austenite phase, as this strongly determines the martensite start temperature, Ms.[33,34] It has been established from a previous study that the optimum quench hardening temperature for the vacuum-sintered X190 steel is 1150 °C.[20] By computational thermodynamics, the carbon content of the austenite phase of the X190 steel at 1150 °C is calculated to be 0.4 wt pct, which is similar to that of the X46 steel substrate (Table I). Hence, it can be suggested that the M7C3 and MC carbide precipitates serving as reinforcement in the sintered X190 are responsible for the difference in its hardness and that of the X46 in the quenched and tempered conditions. The GX20 steel substrate had the lowest hardness of 533 ± 4 HV10, which may be attributed to its low global carbon content (Table I). After tempering at 200 °C for 2 hours, the hardness of all the steels reduced, owing to the relief of the internal stress in the steel post-quench-hardening. Moreover, tempering at low temperatures allows the hardness and strength of the martensitic matrix to be maintained with an increase in toughness.[23,35]
Owing to the interdiffusion across the interface of the steel claddings resulting in microstructural modification, the hardness of this region was measured with a load of 4.9 N (0.5 kg) applied. Figure 12(a) depicts the hardness profile across the interface of the steel claddings after heat treatment. The hardness of the sintered X190 steel is significantly enhanced compared to its as-sintered condition ranging between 700 and 800 HV0.5. In addition, the hardness of the steel substrates X46 and GX20 ranges between 600 and 700 HV0.5. However, low hardness was measured at the near interface region of the X190/GX20 steel cladding but was not apparently observed at the interface region of the X190/X46 cladding. Since the M7C3 carbide-free zone at the X190/X46 cladding is narrow, the hardness of the region with a high volume of retained austenite may not have been measured. At 100 µm away from the interface with GX20, the hardness of X190 was measured to be < 700 HV0.5 with 454 ± 24 HV0.5 measured in X190 at 20 µm distance from the interface. Despite the martensitic matrix in these regions, the hardness is lower owing to no M7C3 carbide precipitates (Figure 8) and a decreasing carbon content due to diffusion into the substrates. The hardness of martensite is known to decrease with decreasing carbon content.[32] As for the GX20 substrate, at 20 µm from the interface, the hardness measured was slightly lower; however, the hardness of the X46 substrate near the interface is slightly higher than the faraway region. With the chemical composition (wt pct) of the austenite phase of the steel claddings at 1150 °C obtained via DICTRA, the martensite start (Ms) temperature across the cladding interface was assessed using Eq. [7].[36]
Figure 12(b) depicts the Ms temperature curves across the interface of X190/X46 and X190/GX20 steel claddings when quenched at 1150 °C. The Ms temperature curves give insight into the hardenability of the steel matrix across the interdiffusion zone at the steel cladding interface. With a focus on the interface region, for the X190/X46 cladding, the Ms temperature of the near interface in X190 is relatively similar to that of the bulk X190. Hence, there is no significant decrease in the hardness of this region despite the presence of no coarse M7C3 carbides. As for the X190/GX20 cladding, Ms temperature decreases slightly in the X190 towards the interface. This indicates the possibility of having more retained austenite in this region, which resulted in a significant decrease in hardness in the X190 towards the interface with GX20.
Table X presents the martensite start temperature of the steel matrices and at their cladding interface based on the empirical formula, which is dependent on the matrix chemical composition. The martensite start temperature of the GX20 steel is the highest and attributed mainly to its lower carbon content compared to the other steels. Thus, the GX20 possesses the lowest fraction of retained austenite after quenching and tempering (Table VII). However, the Ms temperature of the X190/GX20 interface is 219 °C, which is lower than that of the X190 and GX20. This indicates the interface region is enriched by carbon and other elements to reduce the Ms temperature as there are fewer carbide precipitates, which should have consumed the carbon out of the matrix. Upon quenching, retained austenite is inevitable as sufficient undercooling to achieve a complete austenite-to-martensite transformation is not possible via the conventional oil quenching. Hence, a significant drop in the hardness at the X190/GX20 interface region is attributed to the volume of retained austenite and martensite with a low carbon content and low hardness. The Ms temperature of the X46 steel is the lowest among the steels as calculated, and this corroborates the XRD result, as it possesses a higher volume fraction of retained austenite than X190 and GX20 (Table VII). At the X190/X46 interface, the Ms temperature is lower than that of the X190 and slightly higher than that of X46. This indicates the enrichment of the interface region by elements capable of decreasing the Ms temperature. Since adequate undercooling may not be achieved at the interface, a slight enrichment of the X46 adjacent to the interface by austenite stabilizing elements is expected to result in a significant volume of retained austenite as seen in Figure 7(d) and in XRD measurement (Table VII). However, the low hardness measured on either side of the interface could suggest a ductile/tough interface, which is considered beneficial to the tool steel claddings in service as a brittle failure at the interface is least expected.
3.7 Shear Strength
The strength of the sintered X190 steel and the interfacial strength of the steel claddings were assessed through shear tests to evaluate their suitability as tools and potential failure mode in service. Figure 13(a) shows the shear strength of the interface of steel claddings and that of the sintered X190 steel. The interface of the X190/GX20 steel cladding had the highest strength of 798 MPa. The sintered X190 steel had a shear strength of 682 MPa, which is higher than the interfacial strength of the X190/X46 steel cladding. The values of shear strength obtained in this study are higher than those reported for high Cr cast iron/low-carbon steel bimetal by Gao et al.[15] but are comparable to those reported by Li et al.[13] The fracture surface of the sintered X190 steel is characterized by oval dimples and cleavage steps as shown in Figure 13(b). This indicates a mixture of dimple rupture and cleavage fracture modes which are associated with ductile and brittle failure in materials.[37] The shallow oval dimples elongated in the direction of the applied shear load indicated that a ductile part of the steel matrix displayed plastic behavior under load. This plastic behavior can be associated with the presence of retained austenite in the steel matrix after heat treatment as determined by XRD in Figure 11. The cleavage steps are associated with the brittle failure of the M7C3 carbides, which exhibited low or no plasticity under shear load.
(a) Shear strength of the X190 steel and the interface of the steel claddings in the quenched and tempered condition. (b) Fracture surface of the X190 steel. (c) Failure path at the near X190/X46 interface. (d) Fracture surface of the failure path in (c). Insert shows magnified view of cleavage steps. (e) Failure path at the near X190/GX20 interface. (f) Fracture surface of the failure path in (e). Insert shows magnified view of dimples and cleavage steps
Figure 13(c) shows the failure path in the X190/X46 steel cladding when subjected to a shear load. It was observed that the failure occurred in the X190 at the boundary of the zone free of M7C3 carbides before the interface line with the X46. The crack initiation and propagation are expected to have occurred in the M7C3 carbides along the path, which is substantiated by the cleavage steps observed at the edge of the failure path in Figure 13(c) and on the fracture surface in Figure 13(d). The matrix of X190 along the fracture path exhibited low plasticity as river patterns indicating the loading direction were observed on the fracture surface. The low plasticity of the matrix is an indication of low retained austenite in this region, which is also corroborated by the hardness profile across the X190/X46 steel cladding in Figure 12(a). At the near X190/X46 interface, the hardness was > 700 HV0.5, unlike that of X190/GX20, which had a hardness drop below 500 HV0.5. Hence, the matrix is hard and exhibited low ductility, which led to predominantly brittle failure and lower shear strength.
As for the X190/GX20 steel cladding after the shear test, the location of the failure path is similar to that of X190/X46. However, the features on the fracture surface indicated a mixture of dimple rupture associated with ductile materials and cleavage fracture modes associated with hard material exhibiting low plasticity as shown in Figure 13(f). The ductile fracture is identified by the oval and shallow dimples stretched in the direction of the applied shear force especially within the steel matrix. Fine MC carbides observed in some of the dimples act as microvoid nucleating sites owing to the difference in the mechanical properties of the matrix and the carbide, thus resulting in the discontinuity of localized strain in the steel matrix. Therefore, the matrix flows plastically around the carbides thus nucleating the microvoids. The growth and coalescence of the microvoids resulted in the dimple rupture and fracture of the surface. The brittle fracture mode is identified by the cleavage facets, especially on the coarse M7C3 carbides. Localized quasi-cleavage features were also observed on the fracture surface, which indicates that the matrix displayed failure associated with both cleavage and plastic deformation characteristics.[38] It is presumed that an effective load transfer occurred between the carbides and the matrix along the fracture path to impede the failure, which may have led to higher shear strength. Besides, the tortuosity of the fracture path cannot be avoided owing to the microstructural inhomogeneity of the sintered X190 steel. This is evident in the micrographs depicted in Figures 13(c) and (e), as the fracture path deviates from a straight path. Thus, the tortuous path consumed some of the energy required to fracture, which results in a higher shear strength.[39,40] Retained austenite in the X190 matrix near the interface with GX20 is suggested to have been responsible for the mixed fracture modes, as low hardness (454 ± 24 HV0.5) was measured in this region as seen in Figure 12(a). There are possibilities that the retained austenite along the failure path may have undergone hardening due to twinning and/or stress-induced martensitic transformation under overload, significantly increasing the shear strength at the interface of X190/GX20 steel cladding.
Hence, in both steel claddings, the elemental interdiffusion across the interface resulted in a strong metallurgical bonding that does not fail under load. Moreover, crack initiation leading to fracture in the X190 near the interface preferentially occurred through the hard in-situ synthesized carbide precipitates. With fairly ductile, quenched and tempered matrix owing to a significant volume of retained austenite in the tool steel near the interface, high interfacial strength of the steel cladding is achievable.
4 Conclusions
The development of steel claddings by the SLPS of high-chromium X190 tool steel powder on two martensitic stainless steel substrates has been successfully investigated. The effect of the difference in the composition of the substrates on the interfacial diffusion bonding, microstructure, hardness and bonding strength was evaluated with the following key conclusions drawn:
-
(1)
The microstructure of the densified X190 steel powder at a temperature of 1280 °C for 30 minutes is characterized by the dispersion of fine MC and coarse M7C3 carbides in a martensitic matrix upon quenching and tempering. This is in good agreement with the computational thermodynamic evaluations.
-
(2)
The interface of the steel claddings exhibited metallurgical bonding associated with elemental interdiffusion. The chemical potential gradient of the elements is identified as the driving force of the diffusion process, which is in good agreement with the coupled thermodynamic-kinetic DICTRA simulations.
-
(3)
A M7C3 carbide-free, banded region in the X190 is established adjacent to the interface with the substrate, and the width of this region is due to intense carbon diffusion, governed by the chemical potential gradient, from the densified X190 into the substrates.
-
(4)
The hardness of the X190 steel is significantly enhanced above those of the substrates when oil quenched at 1150 °C and tempered at 200 °C for 2 hours. However, the hardness of the steel substrates near the interface is low because of an increase in the volume of retained austenite associated with the carbon enrichment by diffusion from X190 cladding.
-
5)
Higher shear strength is achieved in the X190 near the interface when the matrix is fairly ductile after heat treatment. A mixed fracture mode of oval dimple rupture and cleavage with localized quasi-cleavage features characterized the fracture surface after the application of a shear overload.
References
Garrison WM, Amuda MOH (2017) Stainless Steels: Martensitic, Reference Module in Materials Science and Materials Engineering. Elsevier, Amsterdam
R. Hill and A.L. Perez: New steels and corrosion-resistant alloys, Trends in Oil and Gas Corrosion Research and Technologies, Woodhead Publisher-Elsevier, 2017, pp. 613–26.
T.B. Hilditch, T. de Souza, P.D. Hodgson: Properties and automotive applications of advanced high-strength steels (AHSS), Welding and Joining of Advanced High Strength Steels (AHSS), Woodhead Publisher-Elsevier, 2015, pp. 9–28.
Y. Murakami: Martensitic stainless steels. Metal Fatigue, Academic Press-Elsevier Limited 2019, pp. 431–51.
M. Blüm, H. Hill, H. Moll, S. Weber, and W. Theisen: J. Mater. Eng. Perform., 2012, vol. 21 (5), pp. 756–63.
P.K. Farayibi, T.E. Abioye, A. Kennedy, and A.T. Clare: J. Manuf. Processes, 2019, vol. 45, pp. 429–37.
T.E. Abioye, A. Medrano-Tellez, P.K. Farayibi, and P.K. Oke: Mater. Manuf. Processes, 2017, vol. 32 (14), pp. 1660–66.
G. Lindwall, J. Flyg, K. Frisk, and O. Sandberg: Metall. Mater. Trans. A, 2011, vol. 42 (5), pp. 1165–72.
M. Eroglu and B. Kurt: Mater. Sci. Technol., 2007, vol. 23 (2), pp. 171–76.
H. Wang and S. Yu: Surf. Coat. Technol., 2017, vol. 319, pp. 182–90.
M. Blüm, W. Theisen, and S. Weber: Metall. Mater. Trans. A, 2018, vol. 49 (10), pp 4991–00.
P.K. Farayibi, M. Blüm, W. Theisen, S. Weber: J. Mater. Eng. Perform., 2019, vol. 28 (3), pp. 1833–47.
Y. Li, J. Gao, N. Xu, P. Li, M. Gong, and W. Tong: J. Mater. Eng. Perform., 2019, vol. 28 (11), pp. 6904–11.
C.K. Kim, S. Lee, and J.-Y. Jung: Metall. Mater. Trans. A, 2006, vol. 37 (3), pp. 633–43.
X. Gao, Z. Jiang, D. Wei, S. Jiao, D. Chen, J. Xu, X. Zhang, and D. Gong: Mater. Des., 2014, vol. 63, pp. 650–57.
Y. Li, M. Gong, K. Wang, P. Li, X. Yang, and W. Tong: Mater. Sci. Eng. A, 2018, 718, 260–66.
M. Stolbchenko, O. Grydin, and M. Schaper: Mater. Manuf. Processes, 2017, vol. 33(7), pp. 727-734.
H. Hill, S. Weber, S. Siebert, S. Huth, and W. Theisen: Metall. Mater. Trans. A, 2010, vol. 41A (3), pp. 686-95.
P. Niederhofer, S. Huth, and W. Theisen: Wear, 2015, vol. 332-33, pp. 1059-69.
P.K. Farayibi, M. Blüm, and S. Weber: Mater. Sci. Eng. A, 2020, vol. 777, pp. 139053.
T. Helander, and J. Ågren: Metall. Mater. Trans. A, 1997, vol. 28 (2), pp. 303–08.
A. Borgenstam, L. Höglund, J. Ågren, and A. Engström (2000) J. Phase Equilib. 21(3), 269-80.
[23] J. Garcia, G. Lindwall, O. Prat, and K. Frisk: Int. J. Refract. Met. Hard Mater., 2011, vol. 29 (2), pp. 256–59.
[24] G. Petzow: Metallographisches, Keramographisches, Plastographisches Ätzen, 6. Überarbeitete, Gerbrüder Borntraeger, Sttuttgart, Germany, 1994, pp. 239.
[25] L. Lutterotti, H. Pillière, C. Fontugne, P. Boullay, and D. Chateigner: J. Appl. Crystallogr., 2019, vol. 52 (3) pp. 587–98.
S. Weber, W. Theisen, F. Castro, and A. Pyzalla: Mater. Sci. Eng. A, 2009, vol. 515 (1-2), pp. 175–82.
[27] K.G.F. Janssens, D. Raabe, E. Kozeschnik, M.A. Miodownik, and B. Nestler: Computational Materials Engineering: An Introduction to Microstructure Evolution, Elsevier Academic Press, 2007, pp. 151-74.
H. Mehrer: Diffusion in Solids: Fundamentals, Methods, Materials, Diffusion-Controlled Processes. Springer, Berlin 2007, pp. 161-89.
Hassend F, Weber S (2019) Steel Res. Int. 91(5), 1900481
[40] G. E. Totten: Steel Heat Treatment – Metallurgy and Technologies, 2nd Edition, Taylor & Francis Group LLC, Boca Raton, FL 2006, p. 96.
A.P. Manfridini, G.C.D. de Godoy, and L. Santos: J. Mater. Res. Technol., 2017, 6(1), 65–70
J. Grosch: Microstructure and Properties of Gas Carburised Steels, Comprehensive Materials Processing, Reference Module in Materials Science and Materials Engineering, 2014, vol. 12, pp. 379-411.
P.K. Farayibi, M. Blüm, and S. Weber: HTM J. Heat Treat. Mater. 2020, 75(1), 48-62.
M. Blüm, J. Conrads, S. Weber, and W. Theisen: HTM J. Heat Treat. Mater. 2014, vol. 69 (5), pp. 273-81.
Krauss G (2016) Deformation and Fracture of Martensite before and after Tempering, Reference Module in Materials Science and Materials Engineering. Elsevier, Amsterdam pp. 1-5.
Ishida K (1995) J. Alloys Compd. 220, 126-31.
P. Kulecki, E. Lichańska, A. Radziszewska, and M. Sułowski: Arch. Metall. Mater., 2016, vol. 61 (3), pp. 1613–22.
ASM Handbook, Volume 12 - Fractography, ASM International, The Materials Information Company, 1992, pp. 34–140.
[38] R. Liang, Y. Ji, S. Wang, and S. Liu: Metals, 2016, vol. 6(8), pp. 1-8.
[39] H. Berns, C. Broeckmann, and D. Weichert: Eng. Fract. Mech., 1997, vol. 58(4), pp. 311–25.
Acknowledgments
P. K. Farayibi acknowledges the Alexander von Humboldt Foundation for the award of Humboldt Research Fellowship at The Chair of New Manufacturing Processes and Materials, The Bergische Unversitat Wuppertal, Solingen.
Funding
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted March 12, 2020.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Farayibi, P.K., Blüm, M. & Weber, S. Hard Cladding by Supersolidus Liquid Phase Sintering: An Experimental and Simulation Study on Martensitic Stainless Steels. Metall Mater Trans A 51, 5818–5835 (2020). https://doi.org/10.1007/s11661-020-05953-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-020-05953-4