Introducing the Parkinson’s KinetiGraph into Routine Parkinson’s Disease Care: A 3-Year Single Centre Experience
Abstract
In an effort to provide timely clinical input for people with Parkinson’s disease (PD) in the face of increasing demand and resource limitation in our UK based service, we introduced remote management in place of clinic appointment, including the use of the Parkinson’s KinetiGraph (PKG™), a wrist-worn device that provides a continuous measure of movement. We evaluated our reporting methods and findings, the nature of unmet need we identified, our treatment recommendations and the degree of their implementation in our patients whose feedback guided our service developments. Our evaluation highlighted opportunities and challenges associated with incorporating digital data into care traditionally delivered via in-person contact.
INTRODUCTION
National standards of care for people with Parkinson’s disease (PD) in the UK recommend clinician review at regular intervals of 6–12 months, with follow-up at 2–3 monthly intervals to assess medication response and titrate dosage, and if needed re-visit the diagnosis [1]. In the face of increasing pressure on healthcare services, the working reality for many, including our own centre, is that patients are often not seen when required. Moreover, it is recognised that clinic appointments capture a snapshot of signs and symptoms that may not be faithful to the patient’s true state [2, 3]: a focused history draws on patient recall, which is often incomplete and necessarily low in granularity, while examination may under or overestimate severity due to the inherent changeability of PD symptoms, and motor fluctuations which can vary daily or even hourly [4]. These issues compound clinical management.
Wearable technologies may help to overcome some of these challenges [5] by allowing for continuous, objective and ecologically valid assessment of patients within their homes, reducing the need for the patient to travel to clinic [6], helping to monitor individual responses to treatment, and providing tailored information to optimize it [7]. Yet despite widespread enthusiasm regarding their potential, uptake and adoption into routine clinical care pathways remains slow.
In our service, patients may wait more than 18 months between appointments, with anticipated high unmet need. We introduced the use of the Food and Drug Administration (FDA) approved Parkinson’s KinetiGraph watch (PKG™; Global Kinetics Corporation (GKC)) routinely within our care pathways, between clinic appointments, based on evidence of enhanced in-clinic decision making in PD care [8, 9]. The wrist-worn device has sufficient memory for 6– 10 days of continuous recording, which when analysed by proprietary cloud-based algorithms, provide scores for relevant movement parameters in PD. The device is programmed with the patient’s medication regime and reminders; the patient can acknowledge taking the medication.
We present our early experience with PKG™ at our movement disorders centre to aid in the identification of unmet need and treatment, as well as patient acceptability, and discuss how this led to refinements and future developments in our service.
METHODS
Between July 2015 and January 2018, we conducted 217 PKG™ recordings between clinic appointments within routine clinical care pathways in newly diagnosed PD patients (NP) and follow-up (FU) patients, at our UK University Hospitals Plymouth NHS Trust centre. Patients who were willing to wear the device, living at home and normally ambulant, without significant comorbidities impacting mobility were consecutively offered the use of the device. In the NP pathway, PKG™ was implemented at 6 months to facilitate treatment titration between the 4 and 12-month visits, and in the FU pathway, PKG™ was offered 3– 6 months following the last clinic appointment, to facilitate treatment titration prior to next in-clinic review at 12– 18 months. Patients were asked to attend a short nurse-led clinic, where PKG™ was explained, programmed with their medication regime and applied, to be returned using stamped, addressed envelopes that we provided. Patients wore it for 6 consecutive days (initially 0500– 2200; 24-hour recording from November 2015).
Routine data was collected on demographics, reason for PKG™ request, PKG™ scores and their clinical interpretation, treatment recommendations and outcomes (when possible), and stored in a database (Excel 2010) populated as a live document by the neurology clinical team initially by free text and then using iteratively developed dropdown menus. At the final iteration, up to 4 reasons, findings, recommendations and outcomes could be captured. The reporting process, carried out by the clinical team (CC, EP, FM), evolved as our use of the technology increased and service demands changed, taking 30 minutes on average. Relevant PKG™ indices [see also, 10] include bradykinesia (BKS) and dyskinesia (DKS) [11] with respective detection thresholds for undertreatment [12], percentage of time with tremor (PTT) [13] and percentage of time immobile (PTI), indicative of time immobile (PTI)) indicative of excessive day time sleepiness [14]. Unmet treatment need was identified using thresholds for under-treated motor state, in combination with clinician visual interpretation of the recording (an example of a PKG™ graph is shown in Fig. 1) Reasons for patients being identified by clinician PKG™ interpretation rather than the thresholds included wearing off evident even within an ‘acceptable’ BKS range and dopa-responsive, peri-dose tremor. Visual interpretation of the 24-hour recording also allowed for detection of overnight sleep disturbance. Treatment recommendations were made based on triangulating the PKG™ findings with information available in the patients’ care records. We introduced and tried out different ways of communicating results to patients, including letter, phone call, PKG™ graphs or reporting proformas. Some patients received more than one type of communication. Clinical teams discussed the report findings with the patients and agreed prioritization for intervention. Where possible, the outcome of these conversations was captured in the spreadsheet as ‘outcome’.
Fig. 1
Example of PKG™ Graph.
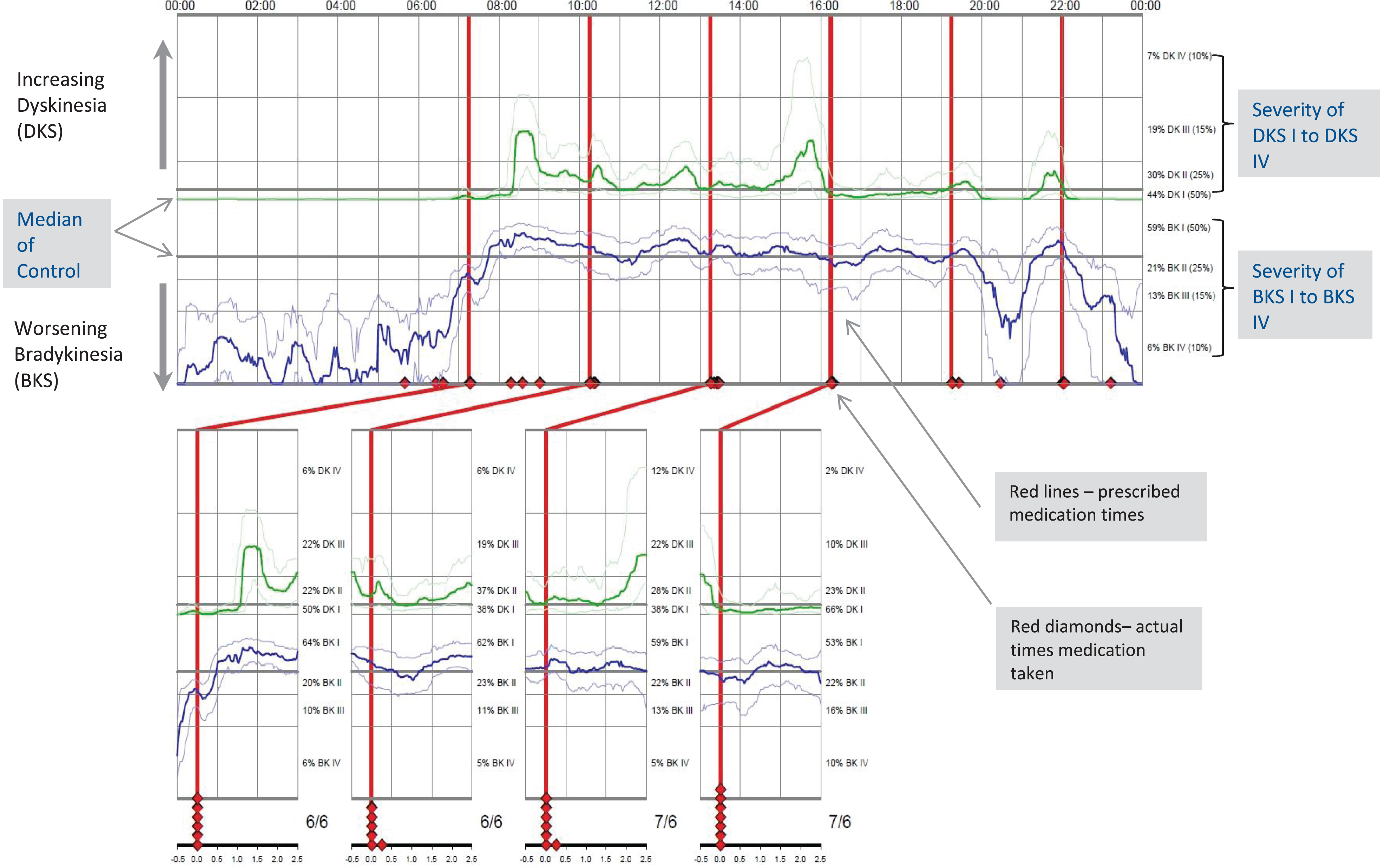
In January 2018, we developed a questionnaire, co-designed with patients who had used PKG™, comprising 24 items related to usability, results communication, satisfaction, along with a freetext response box for concerns and additional comments (Supplementary Material). Questionnaires were posted to the 100 most recent PKG™ users. Scores of 4 and 5 were considered favourable for 5-point items. Freetext responses were thematically examined.
RESULTS
Of the 217 PKG™ recordings, 166 complete datasets from individual patients were included in our service evaluation (88 FU and 78 NP) (characteristics in Table 1), as four were hampered by device failure and four by patient error (e.g., off wrist for long periods), seven were incomplete, and 36 were repeat PKGs™ (to detect changes following an intervention). Further, 62/100 patients (median age 71 years, range 39– 98) returned their evaluation questionnaires.
Table 1
Patient demographics and PKG™ data in the Follow-up (FU) and New Patient (NP) care pathways, displayed as median (range). As the data were not normally distributed, p values refer to non-parametric Mann-Whitney U Test comparisons
| FU (n = 88) | NP (n = 78) | p | |
| Age (y) | 71 (46–85) | 69 (39–87) | 0.99 |
| Gender | 50 F: 38 M | 21 F: 57 M | |
| Disease Duration | 6 y (4 m–23 y) | 1 y (2 m–13 y†) | <0.001 |
| LEDD (mg) | 750 (0–2674) | 375 (0–1000) | <0.001 |
| BKS | 27.2 (6.9–55.9) | 29.6 (15.9–40.5) | 0.39 |
| DKS | 1.9 (0.10–60.4) | 1 (0.1–11.2) | 0.025 |
| FDS | 7.6 (3.8–31.4) | 6.9 (4–17) | 0.089 |
| PTT | 1.35 (0–50.1) | 3.1 (0.1–40.2) | 0.060 |
| PTI | 5.4 (0.1–48.9) | 9.4 (0.3–35.4) | 0.012 |
BKS, bradykinesia score; DKS, dyskinesia score, which maps onto the Abnormal Involuntary Movement Score of excessive movement; FDS, Fluctuation and dyskinesia score, which refers to the range in fluctuation; PTT, percentage of time with tremor; PTI, percentage of time immobile. †As the New Patient (NP) pathway initially included some patients with long standing PD who were newly referred to our service, some patients in this pathway had a disease duration of >1 y.
Unmet need and clinical management
Unmet need relating to different movement parameters was identified in both FU and NP patients (Table 2). The most frequently reported findings in both the FU and NP pathways were of bradykinesia (63% and 72%, respectively) and sleep disturbance (58% and 41%, respectively). Treatment recommendations were made by reporters for 152/166 (92%) patients, with the most common changes relating to dopamine replacement and advice on sleep hygiene and bowel management, for example upon detection of dose failure. Final treatment outcomes obtained retrospectively from follow-up letters were available for 133/166 reports (80%). Treatment recommendations were implemented for 83/114 (73%) patients, with advanced therapy in 6/9 (67%), additional motor agent in 34/71 (48%) and additional non-motor agent in 16/28 (57%). Implementation was guided by patient need. For example 54 patients had ‘wearing off’ identified as a finding, for whom final outcomes were available for 42. Of these 42, 31 (74%) had a change in dopamine replacement therapy to address wearing off, 7 (17%) had no change following discussion with the patient, and 3 (7%) had therapy changes to address another finding (e.g., dyskinesia).
Table 2
The number (percentage) of PKG™ findings as reported by the clinician in the Follow-up and New Patient pathways
| PKG™ Findings | Follow-Up | New Patient |
| (N = 88) | (N = 78) | |
| Bradykinesia | 55 (63%) | 56 (72%) |
| Dyskinesia | 15 (17%) | 3 (4%) |
| Sleep fragmentation* | 46 (58% of 80) | 32 (41%) |
| Daytime somnolence | 16 (18%) | 25 (32%) |
| Prevalent tremor** | 16 (34% of 47) | 29 (40% of 72) |
| Wearing off | 40 (45%) | 14 (18%) |
| Delayed “ON” | 16 (18%) | 0 |
| No clear drug response | 14 (16%) | 7 (9%) |
*Note: Overnight recording became available in November 2015; it is was not available for 8 patients in the FU pathway. **Tremor recording became available in August 2016; it was not available for 41 patients in the FU pathway and 6 patients in the NP pathway.
Patient evaluation
The introductory information and instructions were deemed helpful by most respondents 49/62 (79%) and 60/61 (98%) found the process of returning the device simple; just one reported technical difficulty. Most patients, 41/51 (80%), valued the medication reminders. Our patients’ overall satisfaction with the way results were communicated indicated differences depending on the method used. Out of those who received one, phone calls were scored favorably by 7/14 (50%), letters by 16/34 respondents (47%), the clinician report by 7/16 (44%) and the PKG™ graph by 8/30 (27%). Notably, 24/40 respondents (60%) perceived the PKG™ results as reflective of their lived experience, and 23/39 patients (59%) rated use of the PKG™ as valuable in providing additional information to their clinical team about their condition that they otherwise could not have provided. The vast majority, 57/59 respondents (97%), were willing to continue using it as part of their management while 19/48 (40%) reported satisfaction at not having to travel to clinic; an additional 21 patients were (44%) neutral on this issue.
The main themes around patients’ concerns in-cluded difficulties understanding and delay in receiving the report, and worries around potential technical problems, and whether PKG™ captured their condition accurately. Due to their reported low confidence in their own PD awareness, they would appreciate triggered contacts with their care team, and reported favourably on these interactions.
DISCUSSION
The adoption and use of wearable technology in routine clinical care pathways is far from commonplace. To the best of our knowledge, we are unique in having embraced it for remote management within our service, in lieu of clinical appointment. This service evaluation focused on our early experience with PKG™ represents a first step in assessing its implementation and impact in our service [15]. By sharing our experience, we hope to provide others with insight into the potential value of this approach and help anticipate avoidable pitfalls. Our evaluation is limited by the prospective, evolving nature of the methodology, both in terms of service provision and data capture. We found that PKG™ identified areas of unmet treatment need between clinics and informed treatment recommendations. Patients evaluated the device as acceptable and usable, but highlighted important aspects of our processes that we can improve upon, namely the manner in which findings are communicated and the breadth of assessment.
We encountered a number of challenges in introducing PKG™ into our developing service. First, robust data management is key. Entries by multiple users into our Excel spreadsheet resulted in data capture errors. We have therefore invested in the development of a Clinical Data Warehouse to streamline the consolidation of data on individual patients from multiple sources. Furthermore, communicating about digital outcomes in a way that is meaningful and useful within and between clinical teams necessitates a shared language and familiarity, which requires time, investment and buy-in that should be anticipated. This is no less important than developing effective ways to communicate about these with patients to enhance their understanding and engagement in their care. To mitigate the demands on clinician time required to interpret and report PKG™, we designed and implemented a reporting template. Moreover, to improve accessibility of these findings for patients, we co-designed with them a patient-facing summary report using lay language.
A corollary of our experience with this technology is that our service is evolving organically around and from it, by virtue of its potential to capture signs and behaviour requiring further targeted assessment, as well as our patients’ attitudes and concerns.
For instance, PKG™ data indicating overnight sleep disturbance, which was highly prevalent in our cohort, are agnostic to its causes which may include nocturia, overnight wearing off or REM sleep behavior disorder. Thus, we now routinely include the Parkinson’s Disease Sleep Scale (PDSS2) [16], along with the Non-Motor Symptoms Questionnaire [17] and PD Questionnaire-8 [18] with all PKG™ requests, to evaluate non-motor symptom burden and quality of life and better guide treatment recommendations based on their interplay with motor symptoms. We are developing these assessments into an app, aimed at a more holistic remote PD assessment.
Two important observations became salient over the course our iterative, evolving experience with patients. First, the articulation of trust they place in the care team to identify changes in their condition that they feel ill-equipped to notice. In line with this, we found considerable untreated bradykinesia in our newly diagnosed patients, not just follow-up patients, and more than half of our questionnaire respondents found their PKG™ data differed from their perception of their condition, suggesting that patients become accustomed to their ‘new normal’ with disease development. Second, patients also expressed worry that lack of in-person contact will result in opportunities for care being missed. Both observations underline the need to empower self-awareness and self-management, and PKG™ may be helpful in this respect. This also sits well with the desire that patients have expressed to be able to request a review when needed [19]. To address this, we have co-designed a set of resources to facilitate self-management, including information and guidance around triggered contact which we are now developing into a new remote-care pathway [19].
In this move towards remote care pathways and the use of digital objective measures to guide clinical decision making, we need to develop means of monitoring non-motor symptoms in addition to motor, and work with patients in developing the services that provide and support their care.
CONFLICT OF INTEREST
CC has been in receipt of consultancy fees and honoraria from GKC. TD received a travel bursary from GKC to attend the International Congress of Parkinson’s Disease and Movement Disorders in 2017. PKG™ Recordings undertaken between July 2015 and April 2016 were provided by GKC as part of a national audit supported by Parkinson’s UK. Recordings undertaken after April 2016 were funded by a licence arrangement with GKC, supported by an unrestricted grant from AbbVie Inc. to Plymouth Hospitals NHS Trust.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-202101
REFERENCES
[1] | National Institute for Health and Care Excellence ((2017) ) Parkinson’s disease in adults: Diagnosis and management, Retrieved from https://www.nice.org.uk/guidance/ng71/evidence/full-guideline-pdf-4538466253 |
[2] | Zach H , Dirkx MF , Pasman JW , Bloem BR , Helmich RC ((2017) ) Cognitive stress reduces the effect of levodopa on Parkinson’s resting tremor. CNS Neurosci Ther 23: , 209–215. |
[3] | Zach H , Dirkx M , Pasman JW , Bloem BR , Helmich RC ((2017) ) The patient’s perspective: The effect of levodopa on Parkinson symptoms. Parkinsonism Relat Disord 35: , 48–54. |
[4] | Stocchi F , Jenner P , Obeso JA ((2010) ) When do levodopa motor fluctuations first appear in Parkinson’s disease? Eur Neurol 63: , 257–266. |
[5] | Rovini E , Maremmani C , Cavallo F ((2017) ) How wearable sensors can support Parkinson’s disease diagnosis and treatment: A systematic review. Front Neurosci 11: , 555. |
[6] | Stamford JA , Schmidt PN , Friedl KE ((2015) ) What engineering technology could do for quality of life in Parkinson’s disease: A review of current needs and opportunities. IEEE J Biomed Health Inform 19: , 1862–1872. |
[7] | Barker RW ((2017) ) Is precision medicine the future of healthcare? Per Med 14: , 459–461. |
[8] | Santiago A , Langston JW , Gandhy R , Dhall R , Brillman S , Rees L , Barlow C ((2019) ) Qualitative evaluation of the personal KinetiGraphTM movement recording system in a Parkinson’s clinic. J Parkinsons Dis 9: , 207–219. |
[9] | Carroll C , Kobylecki C , Silverdale M , Thomas C , PKG audit group ((2019) ) Impact of quantitative assessment of Parkinson’s disease-associated symptoms using wearable technology on treatment decisions. J Parkinsons Dis 9: , 601. |
[10] | Pahwa R , Bergquist F , Horne M , Minshall ME ((2020) ) Objective measurement in Parkinson’s disease: A descriptive analysis of Parkinson’s symptom scores from a large population of patients across the world using the Personal KinetiGraph(R). J Clin Mov Disord 7: , 5. |
[11] | Griffiths RI , Kotschet K , Arfon S , Xu ZM , Johnson W , Drago J , Evans A , Kempster P , Raghav S , Horne MK ((2012) ) Automated assessment of bradykinesia and dyskinesia in Parkinson’s disease. J Parkinsons Dis 2: , 47–55. |
[12] | Odin P , Chaudhuri KR , Volkmann J , Antonini A , Storch A , Dietrichs E , Pirtosek Z , Henriksen T , Horne M , Devos D , Bergquist F ((2018) ) Viewpoint and practical recommendations from a movement disorder specialist panel on objective measurement in the clinical management of Parkinson’s disease. NPJ Parkinsons Dis 4: , 14. |
[13] | Braybrook M , O’Connor S , Churchward P , Perera T , Farzanehfar P , Horne M ((2016) ) An ambulatory tremor score for Parkinson’s disease. J Parkinsons Dis 6: , 723–731. |
[14] | Kotschet K , Johnson W , McGregor S , Kettlewell J , Kyoong A , O’Driscoll DM , Turton AR , Griffiths RI , Horne MK ((2014) ) Daytime sleep in Parkinson’s disease measured by episodes of immobility. Parkinsonism Relat Disord 20: , 578–583. |
[15] | NHS England ((2005) ) Institute for Innovation and Improvement, Retrieved from, https://www.england.nhs.uk/improvement-hub/wp-content/uploads/sites/44/2017/11/ILG-1.5-Evaluating-Improvement.pdf |
[16] | Trenkwalder C , Kohnen R , Hogl B , Metta V , Sixel-Doring F , Frauscher B , Hulsmann J , Martinez-Martin P , Chaudhuri KR ((2011) ) Parkinson’s disease sleep scale–validation of the revised version PDSS-2. Mov Disord 26: , 644–652. |
[17] | Chaudhuri KR , Martinez-Martin P , Schapira AH , Stocchi F , Sethi K , Odin P , Brown RG , Koller W , Barone P , MacPhee G , Kelly L , Rabey M , MacMahon D , Thomas S , Ondo W , Rye D , Forbes A , Tluk S , Dhawan V , Bowron A , Williams AJ , Olanow CW ((2006) ) International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: The NMSQuest study. Mov Disord 21: , 916–923. |
[18] | Jenkinson C , Fitzpatrick R ((2007) ) Cross-cultural evaluation of the short form 8-item Parkinson’s Disease Questionnaire (PDQ-8): Results from America, Canada, Japan, Italy and Spain. Parkinsonism Relat Disord 13: , 22–28. |
[19] | Langley J , Ankeny U , Partiridge R , Wheeler G , Carroll C ((2019) ) Co-designing resources for knowledgebased self-reflection for people living with Parkinson’s disease to better enable independent living. In IADE/UNIDCOM’s 10th International Conference Senses & Sensibility 2019: Lost in (G)localization,Lisbon, 27 Nov 2019 - 29 Nov 2019. Springer. |



