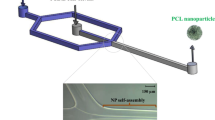
Abstract
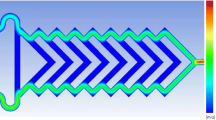
This work aimed at comparing two different microfluidic-assisted nanoparticles (NPs) based on poly(ɛ-caprolactone) (PCL) and cellulose acetate (CA) in terms of size, size distribution, and morphology by changing the flow rate ratios and surfactants. At the same polymer solutions concentration [0.05 (%w/v)], the DLS and FE-SEM results showed that CA NPs have the average diameter (~ 37 nm) and PDI (0.035) less than PCL ones. It was also found that the change of surface tension between the polymers and non-solvent phases using poly(vinyl alcohol) or Tween 80 could remarkably increase the diameter of PCL NPs. Finally, the impact of channel length as a function of mixing efficiency on the size of NPs was theoretically discussed via Reynolds and Peclet numbers. The results indicated that the Peclet numbers pertaining to the required length and time of mixing were more than 100, leading to the smaller channel length for effective mixing.
Graphic abstract








Similar content being viewed by others
References
Leung MHM, Shen AQ (2018) Microfluidic assisted nanoprecipitation of PLGA nanoparticles for curcumin delivery to leukemia jurkat cells. Langmuir 34(13):3961–3970
Chen R, Wulff JE, Moffitt MG (2018) Microfluidic processing approach to controlling drug delivery properties of curcumin-loaded block copolymer nanoparticles. Mol Pharmaceut 15(10):4517–4528
Putro JN, Ismadji S, Gunarto C, Soetaredjo FE, Ju YH (2019) Effect of natural and synthetic surfactants on polysaccharide nanoparticles: hydrophobic drug loading, release, and cytotoxic studies. Colloid Surface A 578:123618
Nam NH, Luong NH (2019) Nanoparticles: synthesis and applications. In: Grumezescu V, Grumezescu A (eds) Materials for biomedical engineering. Elsevier, London, pp 211–240
Łukasiewicz S, Mikołajczyk A, Szczęch M, Szczepanowicz K, Warszyński P, Dziedzicka-Wasylewska M (2019) Encapsulation of clozapine into polycaprolactone nanoparticles as a promising strategy of the novel nanoformulation of the active compound. J Nanopart Res 21(7):149
Najafipour A, Mahdavian AR, Aliabadi HS, Fassihi A (2019) Dual thermo-and pH-responsive poly (N-isopropylacrylamide-co-(2-dimethylamino) ethyl methacrylate)-g-PEG nanoparticle system and its potential in controlled drug release. Polym Bull 77(6):3129–3142
Pattekari P, Zheng Z, Zhang X, Levchenko T, Torchilin V, Lvov Y (2011) Top-down and bottom-up approaches in production of aqueous nanocolloids of low solubility drug paclitaxel. Phys Chem Chem Phys 13(19):9014–9019
Devissaguet JP, Fessi H, Puisieux F (1991) Process for the preparation of dispersible colloidal systems of a substance in the form of nanocapsules. USA Patent
Bilati U, Allémann E, Doelker E (2005) Development of a nanoprecipitation method intended for the entrapment of hydrophilic drugs into nanoparticles. Eur J Pharm Sci 24(1):67–75
Chorny M, Fishbein I, Danenberg HD, Golomb G (2002) Lipophilic drug loaded nanospheres prepared by nanoprecipitation: effect of formulation variables on size, drug recovery and release kinetics. J Control Release 83(3):389–400
Sengel-Turk CT, Ozmen N, Bakar-Ates F (2020) Design, characterization and evaluation of cucurbitacin B-loaded core–shell-type hybrid nano-sized particles using DoE approach. Polym Bull. https://doi.org/10.1007/s00289-020-03256-7
Badri W, Miladi K, Nazari QA, Fessi H, Elaissari A (2017) Effect of process and formulation parameters on polycaprolactone nanoparticles prepared by solvent displacement. Colloid Surf A 516:238–244
Lepeltier E, Bourgaux C, Couvreur P (2014) Nanoprecipitation and the “Ouzo effect”: Application to drug delivery devices. Adv Drug Deliv Rev 71:86–97
Schubert S, Delaney JT Jr, Schubert US (2011) Nanoprecipitation and nanoformulation of polymers: from history to powerful possibilities beyond poly (lactic acid). Soft Matter 7(5):1581–1588
Abstiens K, Goepferich AM (2019) Microfluidic manufacturing improves polydispersity of multicomponent polymeric nanoparticles. J Drug Deliv Sci Technol 49:433–439
Chiesa E, Dorati R, Modena T, Conti B, Genta I (2018) Multivariate analysis for the optimization of microfluidics-assisted nanoprecipitation method intended for the loading of small hydrophilic drugs into PLGA nanoparticles. Int J Pharm 536(1):165–177
Shamsi M, Zahedi P, Ghourchian H, Minaeian S (2017) Microfluidic-aided fabrication of nanoparticles blend based on chitosan for a transdermal multidrug delivery application. Int J Biol Macromol 99:433–442
Karnik R, Gu F, Basto P, Cannizzaro C, Dean L, Kyei-Manu W, Langer R, Farokhzad OC (2008) Microfluidic platform for controlled synthesis of polymeric nanoparticles. Nano Lett 8(9):2906–2912
Khan IU, Serra CA, Anton N, Vandamme TF (2015) Production of nanoparticle drug delivery systems with microfluidics tools. Expert Opin Drug Deliv 12(4):547–562
Russo M, Bevilacqua P, Netti PA, Torino E (2016) A microfluidic platform to design crosslinked hyaluronic acid nanoparticles (cHANPs) for enhanced MRI. Sci Rep-UK 6:37906
Taddei C, Sansone L, Ausanio G, Iannotti V, Pepe GP, Giordano M, Serra CA (2019) Fabrication of polystyrene-encapsulated magnetic iron oxide nanoparticles via batch and microfluidic-assisted production. Colloid Polym Sci 297(6):861–870
Ward K, Fan ZH (2015) Mixing in microfluidic devices and enhancement methods. J Micromech Microeng 25(9):094001
Lee CY, Chang CL, Wang YN, Fu LM (2011) Microfluidic mixing: a review. Int J Mol Sci 12(5):3263–3287
Aubin J, Ferrando M, Jiricny V (2010) Current methods for characterising mixing and flow in microchannels. Chem Eng Sci 65(6):2065–2093
Karnik R (2015) Microfluidic mixing. In: Li D (ed) Encyclopedia of microfluidics and nanofluidics, 2nd edn. Springer, Heidelberg, pp 1969–1979
Guhagarkar SA, Malshe VC, Devarajan PV (2009) Nanoparticles of polyethylene sebacate: a new biodegradable polymer. AAPS PharmSciTech 10(3):935–942
Romanowsky MB, Abate AR, Rotem A, Holtze C, Weitz DA (2012) High throughput production of single core double emulsions in a parallelized microfluidic device. Lab Chip 12(4):802–807
Vladisavljević GT, Shahmohamadi H, Das DB, Ekanem EE, Tauanov Z, Sharma L (2014) Glass capillary microfluidics for production of monodispersed poly (DL-lactic acid) and polycaprolactone microparticles: Experiments and numerical simulations. J Colloid Interface Sci 418:163–170
Majedi FS, Hasani-Sadrabadi MM, VanDersarl JJ, Mokarram N, Hojjati-Emami S, Dashtimoghadam E, Bonakdar S, Shokrgozar MA, Bertsch A, Renaud P (2014) On-chip fabrication of paclitaxel-loaded chitosan nanoparticles for cancer therapeutics. Adv Funct Mater 24(4):432–441
Carrick C, Larsson PA, Brismar H, Aidun C, Wågberg L (2014) Native and functionalized micrometre-sized cellulose capsules prepared by microfluidic flow focusing. RSC Adv 4(37):19061–19067
Edgar KJ (2007) Cellulose esters in drug delivery. Cellulose 14(1):49–64
Hornig S, Heinze T (2008) Efficient approach to design stable water-dispersible nanoparticles of hydrophobic cellulose esters. Biomacromol 9(5):1487–1492
Kulterer MR, Reichel VE, Kargl R, Köstler S, Sarbova V, Heinze T, Stana-Kleinschek K, Ribitsch V (2012) Functional polysaccharide composite nanoparticles from cellulose acetate and potential applications. Adv Funct Mater 22(8):1749–1758
Peng B, Almeqdadi M, Laroche F, Palantavida S, Dokukin M, Roper J, Yilmaz OH, Feng H, Sokolov I (2019) Ultrabright fluorescent cellulose acetate nanoparticles for imaging tumors through systemic and topical applications. Mater Today 23:16–25
Kulterer MR, Reischl M, Reichel VE, Hribernik S, Wu M, Köstler S, Kargl R, Ribitsch V (2011) Nanoprecipitation of cellulose acetate using solvent/nonsolvent mixtures as dispersive media. Colloid Surf A 375(1–3):23–29
Wondraczek H, Petzold-Welcke K, Fardim P, Heinze T (2013) Nanoparticles from conventional cellulose esters: evaluation of preparation methods. Cellulose 20(2):751–760
Bordes C, Fréville V, Ruffin E, Marote P, Gauvrit JY, Briançon S, Lantéri P (2010) Determination of poly (ɛ-caprolactone) solubility parameters: application to solvent substitution in a microencapsulation process. Int J Pharm 383(1–2):236–243
Nair LS, Laurencin CT (2007) Biodegradable polymers as biomaterials. Prog Polym Sci 32(8–9):762–798
Lee JS, Hwang SJ, Lee DS, Kim SC, Kim DJ (2009) Formation of poly (ethylene glycol)-poly (ε-caprolactone) nanoparticles via nanoprecipitation. Macromol Res 17(2):72–78
Roldán GJC, Gomez LMA, Cornelio JAC, Rodriguez LF, Pinal R, Palacio LMH (2018) Production of polycaprolactone nanoparticles with low polydispersity index in a tubular recirculating system by using a multifactorial design of experiments. J Nanopart Res 20(3):68
Badri W, Miladi K, Robin S, Viennet C, Nazari QA, Agusti G, Fessi H, Elaissari A (2017) Polycaprolactone based nanoparticles loaded with indomethacin for anti-inflammatory therapy: from preparation to ex vivo study. Pharm Res 34(9):1773–1783
Othman R, Vladisavljević GT, Nagy ZK (2015) Preparation of biodegradable polymeric nanoparticles for pharmaceutical applications using glass capillary microfluidics. Chem Eng Sci 137:119–130
Kwon HJ, Kim S, Kim S, Kim JH, Lim G (2017) Controlled production of monodisperse polycaprolactone microspheres using flow-focusing microfluidic device. BioChip J 11(3):214–218
Lallana E, Donno R, Magrì D, Barker K, Nazir Z, Treacher K, Lawrence MJ, Ashford M, Tirelli N (2018) Microfluidic-assisted nanoprecipitation of (PEGylated) poly (d, l-lactic acid-co-caprolactone): effect of macromolecular and microfluidic parameters on particle size and paclitaxel encapsulation. Int J Pharm 548(1):530–539
Othman R, Vladisavljevic GT, Nagy ZK, Holdich RG (2016) Encapsulation and controlled release of rapamycin from polycaprolactone nanoparticles prepared by membrane micromixing combined with antisolvent precipitation. Langmuir 32(41):10685–10693
Witzigmann D, Sieber S, Porta F, Grossen P, Bieri A, Strelnikova N, Pfohl T, Prescianotto-Baschong C, Huwyler J (2015) Formation of lipid and polymer based gold nanohybrids using a nanoreactor approach. RSC Adv 5(91):74320–74328
Romoli L, Tantussi G, Dini G (2011) Experimental approach to the laser machining of PMMA substrates for the fabrication of microfluidic devices. Opt Lasers Eng 49(3):419–427
Dirksen JA, Ring TA (1991) Fundamentals of crystallization: kinetic effects on particle size distributions and morphology. Chem Eng Sci 46(10):2389–2427
Zhao H, Wang JX, Wang QA, Chen JF, Yun J (2007) Controlled liquid antisolvent precipitation of hydrophobic pharmaceutical nanoparticles in a microchannel reactor. Ind Eng Chem Res 46(24):8229–8235
Hansen CM (2007) Hansen solubility parameters: a User's handbook, 2nd edn. CRC Press, New York
Sharratt WN, Brooker A, Robles ESJ, Cabral JT (2018) Microfluidic solvent extraction of poly (vinyl alcohol) droplets: effect of polymer structure on particle and capsule formation. Soft Matter 14(22):4453–4463
Tsukada Y, Hara K, Bando Y, Huang C, Kousaka Y, Kawashima Y, Morishita R, Tsujimoto H (2009) Particle size control of poly (dl-lactide-co-glycolide) nanospheres for sterile applications. Int J Pharm 370(1–2):196–201
Pustulka KM, Wohl AR, Lee HS, Michel AR, Han J, Hoye TR, McCormick AV, Panyam J, Macosko CW (2013) Flash nanoprecipitation: particle structure and stability. Mol Pharm 10(11):4367–4377
Hapse SA, Rachh PR (2019) Nanotechnology based approaches for enhancements of bioavailability of sustain release formulation. J Drug Deliv Ther 9(3):617–625
Tammaro O, di Polidoro AC, Romano E, Netti PA, Torino E (2020) A microfluidic platform to design multimodal PEG-crosslinked hyaluronic acid nanoparticles (PEG-cHANPs) for diagnostic applications. Sci Rep-UK 10(1):6028
Wongpinyochit T, Totten JD, Johnston BF, Seib FP (2019) Microfluidic-assisted silk nanoparticle tuning. Nanoscale Adv 1(2):873–883
Xie H, Smith JW (2010) Fabrication of PLGA nanoparticles with a fluidic nanoprecipitation system. J Nanobiotechnol 8(1):18
Campardelli R, Della Porta G, Reverchon E (2012) Solvent elimination from polymer nanoparticle suspensions by continuous supercritical extraction. J Supercrit Fluid 70:100–105
Nowak E, Kovalchuk NM, Che Z, Simmons MJH (2016) Effect of surfactant concentration and viscosity of outer phase during the coalescence of a surfactant-laden drop with a surfactant-free drop. Colloid Surf A 505:124–131
Budhian A, Siegel SJ, Winey KI (2007) Haloperidol-loaded PLGA nanoparticles: systematic study of particle size and drug content. Int J Pharm 336(2):367–375
Poletto FS, Fiel LA, Donida B, Ré MI, Guterres SS, Pohlmann AR (2008) Controlling the size of poly (hydroxybutyrate-co-hydroxyvalerate) nanoparticles prepared by emulsification–diffusion technique using ethanol as surface agent. Colloid Surf A 324(1–3):105–112
Ray S, Mishra A, Mandal TK, Sa B, Chakraborty J (2015) Optimization of the process parameters for the fabrication of a polymer coated layered double hydroxide-methotrexate nanohybrid for the possible treatment of osteosarcoma. RSC Adv 5(124):102574–102592
Doolaanea AA, Ismail AFH, Nor NHM, Mohamed F (2015) Effect of surfactants on plasmid DNA stability and release from poly (D, L-lactide-co-glycolide) microspheres. Trop J Pharm Res 14(10):1769–1778
Bayareh M, Ashani MN, Usefian A (2020) Active and passive micromixers: A comprehensive review. Chem Eng Process 147:107771
Sakurai R, Yamamoto K, Motosuke M (2019) Concentration-adjustable micromixers using droplet injection into a microchannel. Analyst 144(8):2780–2787
Zhang Z, Zhao P, Xiao G, Lin M, Cao X (2008) Focusing-enhanced mixing in microfluidic channels. Biomicrofluidics 2(1):014101
Lim TW, Son Y, Jeong YJ, Yang DY, Kong HJ, Lee KS, Kim DP (2011) Three-dimensionally crossing manifold micro-mixer for fast mixing in a short channel length. Lab Chip 11(1):100–103
Rasouli MR, Abouei Mehrizi A, Lashkaripour A (2015) Numerical study on low Reynolds mixing oft-shaped micro-mixers with obstacles. Transp Phenom Nano Micro Scales 3(2):68–76
Acknowledgements
The authors would like to express their sincere thanks from the research council of University of Tehran for the experimental services in this work.
Funding
There is no funding organization for carrying out the experiments of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lari, A.S., Khatibi, A., Zahedi, P. et al. Microfluidic-assisted production of poly(ɛ-caprolactone) and cellulose acetate nanoparticles: effects of polymers, surfactants, and flow rate ratios. Polym. Bull. 78, 5449–5466 (2021). https://doi.org/10.1007/s00289-020-03367-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03367-1