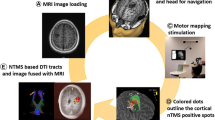
Abstract
The objective of this systematic review is to create an overview of the literature on the comparison of navigated transcranial magnetic stimulation (nTMS) as a mapping tool to the current gold standard, which is (intraoperative) direct cortical stimulation (DCS) mapping. A search in the databases of PubMed, EMBASE, and Web of Science was performed. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and recommendations were used. Thirty-five publications were included in the review, describing a total of 552 patients. All studies concerned either mapping of motor or language function. No comparative data for nTMS and DCS for other neurological functions were found. For motor mapping, the distances between the cortical representation of the different muscle groups identified by nTMS and DCS varied between 2 and 16 mm. Regarding mapping of language function, solely an object naming task was performed in the comparative studies on nTMS and DCS. Sensitivity and specificity ranged from 10 to 100% and 13.3–98%, respectively, when nTMS language mapping was compared with DCS mapping. The positive predictive value (PPV) and negative predictive value (NPV) ranged from 17 to 75% and 57–100% respectively. The available evidence for nTMS as a mapping modality for motor and language function is discussed.
Similar content being viewed by others
Introduction
In neurosurgical practice, often lesions are encountered, which are invading into eloquent brain regions or have a close relation with eloquent brain structures (i.e., motor, language, or other cognitive function). This is true for glioma surgery, for different kinds of vascular surgery, epilepsy surgery, and for surgical procedures aiming at resection of lesions like metastasis, meningiomas, and cavernomas. To maximize the safety and extent of resection of neurosurgical procedures, multiple mapping and monitoring modalities have been developed. The review of Ottenhausen et al. [37] gives a good overview of the available techniques. Besides nTMS, magnetoencephalography (MEG), diffusion tensor imaging-fiber tracking (DTI-FT), and functional magnetic resonance imaging (fMRI) are available for preoperative mapping. In the intraoperative setting, DCS mapping and monitoring and subcortical stimulation (SCS) mapping are useful techniques. MEG records neuronal activity by measuring magnetic fields produced by electric currents in the brain. With the MEG technique, spatiotemporal mapping of motor, somatosensory, language, auditory, and visual functions can be performed [37]. The high cost of MEG, and therefore the limited availability, is a big disadvantage of this technique. DTI-FT enables subcortical white matter fiber tract delineation. However, it is a purely radiologic anatomical imaging technique that does not include physiological functional data [37]. Traditionally, fMRI is the most available and employed preoperative mapping technique for localization of eloquent cortical brain areas for different types of neurological functions. One of the problems of the fMRI technique is that blood-oxygen-level-dependent (BOLD) imaging shows blood oxygenation level as a surrogate parameter of neuronal activity. It cannot be discerned if a BOLD signal represents a critical cortical area or only a participatory, non-essential cortical area for the tested neurological function. The performance of fMRI is suboptimal in the vicinity of brain tumors [10]. Regarding sensitivity and specificity of fMRI motor function localization, the literature is not unambiguous [37]. Also, fMRI language localization sensitivity and specificity show a very broad range, limiting its ability as a presurgical mapping tool [13]. In general, only fMRI mapping in adjunct to other methods is advised [37].
In recent years, many studies on the use of navigated transcranial magnetic stimulation (nTMS) as a preoperative mapping tool have been published. nTMS enables inhibition or excitation of a cortical area by way of repetitive or single transcranial magnetic pulses, respectively. This technique is appealing, because it can be conducted preoperatively in a controlled environment. The procedure can be repeated as often as is deemed necessary by the treating physician. This in contrary to intraoperative direct cortical stimulation (DCS) mapping, where fatigue and loss of optimal concentration during awake procedures, epileptic seizures as well as duration of the surgery can be limiting factors for the mapping procedure. Furthermore, the cortical surface that can be mapped intraoperatively is limited to the extent of exposure of brain cortex by the craniotomy. Nonetheless, intraoperative mapping is still considered the gold standard amongst most neurosurgeons.
The aim of this review is to give a contemporary overview of the existing literature comparing nTMS mapping to DCS mapping techniques. In the previous literature, a smaller scale review from Takahashi et al. [53] on localization of motor function by way of nTMS appeared in 2013, in which 11 articles were included. The recent review and meta-analysis of Raffa et al. [46] from 2019 also focuses solely on motor mapping, with an emphasis on the effect on oncologic treatment outcome, and is not informative concerning the comparison of nTMS and DCS mapping. In this publication, the authors found a significantly reduced risk of postoperative new permanent motor deficit and an increased rate of gross-total resection (GTR), in favor of the use of nTMS. Also, a smaller craniotomy size and a trend toward a reduction in the duration of the surgery were found in this meta-analysis. However, the authors also conclude that there is a need for high-level evidence from multicenter randomized controlled studies. Our review adds to the abovementioned literature by considering not only motor mapping but also language mapping. For other neurological functions, no comparative data could be found at this moment.
Materials and methods
The primary research objective was formulated as follows: the comparison of mapping techniques in patients with a neurosurgical intervention in an eloquent brain area (population) undergoing preoperative cortical mapping using nTMS and cortical mapping by DCS for neurological function localization (outcome) in prospective and retrospective comparative case series and cohort studies (study design).
Search strategy
On 17 September 2019, a literature search was performed in the electronic databases of PubMed, EMBASE, and Web of Science. A search strategy was formulated for each of the three databases (see Table 1 for the PubMed search strategy; the comparable search strings for all three databases are given in Supplement 1). The review was conducted according to the PRISMA guidelines and recommendations.
Study selection criteria
The steps of selection of the articles for inclusion are shown in the flowchart (Fig. 1). Articles that met the following criteria were included: (1) articles describing patients undergoing both a nTMS mapping procedure and a DCS mapping procedure. (2) A comparison in function localization between both mapping techniques was performed. The study selection procedure was performed by three of the authors (H-RJ, A-KO, AW). Each citation was checked by at least two different researchers. Disagreement was resolved by discussion. The following languages were allowed for inclusion: English, German, French, Italian, and Spanish. There was no restriction in the type of neurological function that was being mapped, although motor and language were the predominant neurological functions investigated in most publications. The references of the articles selected for full-text reading were hand searched for new eligible citations. This did not lead to the addition of any new citations. Comments, letters to the editor, and author replies were excluded because they contained no new experimental data. Case reports describing a single patient were excluded in this review.
Quality assessment of included articles
The study design of the included articles in this review was noted. Furthermore, the articles were scored on four different domains as described by Murad et al. [33] in a modified way. For every domain, the information in a publication was evaluated as good, moderate, or insufficient. The four domains were patient selection (Do the patients represent the whole experience of the investigator/center? Is the selection method unclear to the extent that other patients with similar presentation may not have been reported?), ascertainment (Were exposure and outcome adequately ascertained?), causality (Were other alternative causes that may explain the outcome ruled out?), and reporting (Are sufficient details given to allow other researchers to replicate the research or make inferences related to their own practice?).
Data extraction from articles
From the available full-text articles, the following information was extracted: information on the comparison of mapping outcome of nTMS and DCS. For motor mapping, in most studies, the Euclidian distance was given between nTMS- and DCS-mapped cortical representations of muscle groups. For language studies, often sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were used to evaluate nTMS mapping, by comparison of nTMS results with “gold standard” DCS mapping results. To obtain these data, most studies divide the cortical surface in many small cortical areas and calculate the (dys)congruence of the mapping results from the nTMS and DCS techniques for each of these areas. If available, the following information was also extracted: the number of patients in the study on which the comparison between nTMS and DCS was made (this did not always correspond to the total number of patients included in a study), the year of publication, and the disease type of the patient population. The type of TMS machine and other hardware used for the preoperative mapping procedure were noted. The nTMS mapping protocol and settings were recorded as completely as possible from the description in the article. A qualitative analysis was performed.
Results
The initial database search yielded 2190 citations. After removal of duplicates and title and abstract screening, 62 citations remained for full-text reading. After the final step in the selection process, 35 articles were included in this review. Study design of the included articles and information regarding the domains described by Murad et al. were determined (Table 2). There were 26 publications with data about motor mapping and 10 publications with data about language mapping (one publication giving information on both motor and language mapping). No publications on the comparison of nTMS mapping and DCS mapping for other neurological functions were found. The publications appeared between 1997 and 2019.
nTMS for motor function
Patient population
Twenty-six articles with information about nTMS and DCS mapping of motor function were found, describing a total of 364 patients (Table 3) [1, 3, 6,7,8, 20,21,22, 24,25,26, 29, 31, 36, 38, 39, 41, 43,44,45, 48, 51, 54, 56, 58, 59]. The technique is used in tumor patients with different kinds of histopathology (high-grade/low-grade glioma, metastasis, meningioma, DNET, lymphoma, hemangiopericytoma, ganglioma, and cavernoma), in patients with vascular lesions, and in epilepsy patients. In all studies, patients tolerated nTMS mapping well. No adverse events were being mentioned.
nTMS motor mapping protocols
Different nTMS protocols were used for motor mapping. In 22 articles, the (resting) motor threshold (R)MT was determined. Two articles only mention the percentage of maximum stimulator output (MSO) that was applied [21, 22]. Another article also used the active motor threshold (AMT) in some patients to determine the stimulator output setting [54]. In the articles where (R)MT was obtained, the stimulation intensity varied from 105 to 130% of (R)MT for mapping of motor function.
Muscle groups mapped with nTMS
In the included studies, hand, arm, leg, and facial muscles are mapped with nTMS. Hand muscles are most often mapped (in all 26 available articles), followed by leg muscles (in 14 out of 26 articles).
Comparison of nTMS and DCS motor mapping
Eighteen articles describe the distance between nTMS-mapped functional points and DCS-mapped functional points as an outcome measure. Average/median (Euclidian) distances of 2.13–16 mm are reported. The data in the publication of Kantelhardt et al. [21] were not taken into consideration here, because the authors give a distance between nTMS mapping and DCS mapping after removal of the tumor. In this situation, most likely brainshift will have occurred. Fourteen articles describe an accuracy of < 10 mm for nTMS motor mapping compared with DCS mapping. Most authors conclude that nTMS motor mapping is reliable compared with DCS mapping.
nTMS devices for motor mapping
For motor mapping, 15 out of 26 articles used a Nexstim navigated brain stimulation (NBS) system (Helsinki, Finland). Two other manufacturers of TMS devices, Magstim (Whitland, UK) and MagVenture (Farum, Denmark), could be identified in the included publications.
nTMS for language function
Patient population
Ten publications give information about language mapping with nTMS and DCS, describing in total 188 patients (Table 4) [2, 18,19,20, 27, 28, 30, 40, 52, 55]. In one of their publications, Ille et al. mention that some patient data have been used in previous studies [19]. The use of patient data in multiple publications could potentially make the total number of patients described in the literature, regarding the comparison of nTMS language mapping and DCS language mapping, lower than the number mentioned here. In the included studies in this review, language function in tumor patients and epilepsy patients was mapped. No adverse events are mentioned in the studies. The nTMS language mapping was also well tolerated in pediatric patients according to Lehtinen et al. [30]. In the study, 14 pediatric and adolescent patients were included, with an age ranging from 9 to 18 years.
nTMS language mapping protocols
For language mapping, repetitive TMS (rTMS) is used to suppress part of the (sub)cortical network responsible for the production of language. All studies use the NexSpeech module of the Nexstim NBS system, in which an object naming task has to be performed. Stimulation was done at 76–120% of resting motor threshold (RMT). In the rTMS mapping protocols, between 5 and 20 TMS bursts were given, with a frequency ranging from 5 to 10 Hz. Other variables in the stimulation protocols were duration of picture display time (range 700 ms–3 s), interpicture interval (range 2–5 s), and picture-to-stimulation interval (range 0 s–500 ms).
DCS language mapping protocols
The DCS language mapping was conducted using the “Penfield technique” in eight studies, with a stimulation frequency of 40–60 Hz during 4 s. In six studies, intraoperative electrocorticography (ECoG) was applied to detect epileptic activity and afterdischarges after stimulation. In the two studies on epilepsy patients, a subdural grid electrode was used for DCS extraoperative language mapping.
Type of language error elicited by stimulation
All articles mention in their study protocols the type of language errors that are registered during the nTMS mapping procedure (speech arrest, performance error, hesitation, neologism, semantic paraphasia, phonologic paraphasia, circumlocution, anomia). Only Sollmann et al. analyzed the correlation between the type of language error that could be evoked with nTMS mapping and the type of error found with DCS mapping [52]. Lehtinen et al. give information about the percentage of true positive nTMS mapped types of language errors in relation to the DCS mapping outcome, which ranged between 14 and 76% [30].
Comparison of nTMS and DCS language mapping
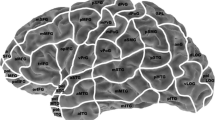
Eight publications use a cortical parcellation system (CPS) for language mapping. In this model, the hemisphere is divided into 37 anatomical regions. The two other studies use the Montreal Neurological Institute (MNI) coordinate system. The comparison between language positive and negative nTMS and DCS points makes calculation of sensitivity, specificity, NPV, and PPV possible. Sensitivity ranged from 10 to 100% and specificity ranged from 13.3 to 98%. NPV and PPV ranged from 57 to 100% and 17–75%, respectively. Cut-off values, regarding when a cortical region is considered positive or negative for language function, strongly influenced these outcomes. The negative mapped areas clearly had the highest predictive value. One study mentioned distance between nTMS- and DCS-mapped points as an outcome measure. Babajani-Feremi et al. [2] described a Euclidian distance of 8.7 mm between nTMS- and DCS-mapped localizations. In four articles, a separate analysis for the posterior and anterior language areas and nTMS mapping accuracy was performed. The anterior (Broca’s) language areas had the most reliable nTMS mapping results. Most articles conclude that nTMS language mapping is clinically useful, especially in regard to negatively mapped regions.
nTMS devices for language mapping
All nTMS language mapping was done with the Nexstim equipment and software. There was no diversity in manufacturer of the device and language testing software.
Discussion
nTMS is a relatively new, promising mapping technique for cortical function localization. This article provides an overview of the available literature on the comparison of nTMS with DCS mapping in the neurosurgical practice. At the moment, comparative data are only available for motor and language mapping. For other modalities (e.g., arithmetic function/calculation, neglect/spatial function, visual field aspects), only non-comparative data in healthy subjects and sometimes patients are available [11, 12, 14, 32, 49]. This renders multiple unresolved questions for future research. Comparing the results of nTMS and DCS mapping for other modalities can help cross-validate the results of the relatively new motor and language literature. The number of centers publishing their data on nTMS mapping is growing but still limited, with some centers being the predominant publishers/collaborators. Especially the Munich and Berlin neurocenters have a broad experience with the nTMS mapping technique and are responsible for 31% of the publications included in this systematic review.
nTMS motor mapping
The largest body of evidence is available for nTMS motor mapping. The technique has proven to be reliable on a scale of millimeters compared with DCS in a large number of studies (Table 3). Hand motor function is the most frequently mapped cortical area. However, other muscles can be mapped with the nTMS technique as well. There is an extensive body of literature on nTMS motor mapping, forming a solid base for its application in clinical practice. Although there is excellent agreement between preoperative nTMS motor mapping and DCS motor mapping, intraoperative monitoring of the pyramidal tract and SCS are still indispensable, to secure integrity of the entire motor pathway.
nTMS language mapping
Language mapping with nTMS has also extensively been described in the literature, albeit to a lesser extent than motor mapping. The technique of language mapping is more complex, because language is the result of a network function, which is more difficult to localize and map than a circumscriptive motor area in the precentral gyrus [47]. In the studies on nTMS language mapping, a notable large variability in sensitivity, specificity, NPV, and PPV was observed. The different values are highly dependent on the criteria that are used to determine if a cortical area is considered positive or negative for language function. In DCS mapping during awake surgery, the 2-out-of-3 rule is commonly applied, to decide if a specific area is language eloquent or not. This rule implicates that a cortical area is stimulated three times. The specific localization will be regarded eloquent for the tested neurological function, if a performance error can be provoked at least two times due to stimulation. If the amount of positive stimulations is less than 2-out-of-3, then the area is regarded non-eloquent for the tested neurological function. The nTMS mapping error rate, which is used as a cut-off value for positive or negative language function localization, greatly influences sensitivity, specificity, NPV, and PPV [19]. For the moment, there are no guidelines available, advising on which error rates should be used as a cut-off in deciding on eloquence versus non-eloquence in nTMS language mapping.
Also, methodological differences influence accuracy results of the included studies, as has been pointed out by Tarapore et al. [54]. For example, their use of a more dense grid to separate different mapped brain regions than other groups and a standardized data normalization algorithm can explain the relatively high sensitivity, specificity, and NPV in their publication. Furthermore, the methodological definition of nTMS/DCS concordance versus nTMS/DCS non-concordance influences the accuracy outcomes.
False-negative nTMS-mapped regions are, of course, a major concern. However, the total number of false-negative nTMS-mapped areas is low in all included studies. The false-negative areas occurred predominantly in the posterior language areas.
Most authors conclude that nTMS language mapping is a very useful preoperative tool, but the technique cannot replace DCS during awake surgery and it should be used as an adjunct to awake intraoperative testing. There is no supportive literature for resection of language-eloquent lesions based on nTMS functional data alone. Only in patients in which an awake procedure is not feasible (e.g., due to a psychiatric condition or in young children), it can be considered to perform a resection without awake testing based on nTMS mapping results (combined with DTI-FT) as has been described by Ille et al. [17]. In their case series of four patients, who did not qualify for awake surgery and had a nTMS-based resection under general anesthesia, no new neurological deficits occurred. However, one patient underwent a second resection several days after the primary procedure to achieve a complete resection. The authors advocate that nTMS-based resection can only form a “rescue strategy” for patients who do not qualify for awake surgery.
nTMS mapping protocols
Regarding nTMS mapping protocols, our findings show that differences in mapping protocols exist. In recent literature, this is especially true for language mapping protocols. For example, the publication from Krieg et al. shows that the timing of nTMS pulse onset after picture presentation influences the nTMS mapping result [28]. Also, differences in the number of TMS bursts and time intervals (interpicture interval, picture-display-time) exist. This opens possibilities for future directions/perspectives. A recent consensus meeting about the protocol for motor and language mapping, however, has helped to overcome major diversity in current practice [23]. During this meeting, participating experts agreed that there is enough supportive evidence for the use of nTMS motor mapping in routine clinical practice. Details on the nTMS motor mapping protocol are given in the supplementary material of the meeting report [23]. In the opinion of this consensus group, nTMS language mapping should be performed in the framework of clinical studies. In the meeting report, a nTMS language mapping protocol is proposed and the parameters are appointed that should be taken into consideration when performing nTMS language mapping. It is stated, however, that further refinement of this protocol is necessary [23]. Optimization of nTMS mapping protocols should be achieved, primarily, by testing different settings in healthy subjects.
Furthermore, there is a need for standardization regarding the interpretation of nTMS responses. Especially cut-off values when a stimulated area is considered positive or negative should become more clearly established in future protocols. Besides, in most nTMS language mapping sessions, only an object naming task was performed. There are no data on nTMS mapping test batteries containing, for example, verb generation, reading, and writing, and the comparison with DCS mapping results of those functions. Also, data regarding the correlation between type of language errors (e.g., speech arrest, anomia, phonemic paraphasia, semantic paraphasia, hesitation) in nTMS and DCS mapping procedures are scarce.
In DCS mapping, ECoG is frequently used intraoperatively to be informed about epileptic activity and afterdischarges following cortical stimulation, which can form an alternative explanation for language errors. The addition of electroencephalography (EEG) to nTMS mapping is not applied in most protocols. The combination of both techniques could possibly make the interpretation of stimulation results in nTMS language mapping more accurate. Although during nTMS mapping hardly any epileptic seizures have been encountered according to the literature, it could be interesting to know if a nTMS-provoked speech disturbance is a very focal effect, or that the stimulation maybe did cause a more widespread disturbance/epileptiform activity in patients than is currently believed.
nTMS mapping devices
The diversity in TMS hardware/devices is limited. Predominantly Nexstim NBS (Helsinki, Finland) machines were used. In total, 24 out of 35 publications use the Nexstim equipment. Two other manufacturers of nTMS devices for mapping of neurological function could be identified in the included articles in this review, which are Magstim (Whitland, UK) and MagVenture (Farum, Denmark).
nTMS mapping and fMRI function localization
nTMS mapping has been compared with fMRI mapping in several studies as well. For motor function localization, there is support that nTMS mapping is more accurate than fMRI [3, 7, 30] and, in addition, the distance between nTMS- and DCS-mapped functional regions is smaller than the distance between nTMS- and fMRI-mapped functional regions [23,24,25]. Regarding mapping of language function, nTMS mapping is more sensitive, but less specific than fMRI [2, 18].
nTMS mapping for other purposes than preoperative cortical function localization
Although initially during its development in the neurosurgical practice, the focus was on nTMS being a preoperative mapping tool, many new applications have been described recently. There is literature describing nTMS as a tool to investigate plasticity and shift of neurological function localization over time in patients suffering from different neurological conditions [15]. With this, nTMS becomes an instrument to judge the possibility of secondary craniotomies after the primary procedure because, due to the plasticity and shift of neurological function, new opportunities for safe resections might become possible in the course of the disease, which were not possible during the primary procedure due to eloquence. Also, nTMS data are successfully used as seeding point for diffusion tensor imaging (DTI) fiber tracking of white matter tracts [4, 9, 34, 35, 60]. There is literature describing nTMS mapping as a helpful tool in the planning of radiosurgery targets in eloquent brain regions [5, 42, 50, 57]. Last but not least, nTMS is used as a method to determine eloquence and thus classification of arteriovenous malformations and, depending on this classification, the treatment of those lesions [16]. All these purposes are useful in clinical practice. However, it must be emphasized that the accuracy of the nTMS technique remains the pivotal mainstay for all aforementioned purposes. The current review gives an overview of the available data concerning this topic.
Study limitations
The data in this systematic review were not deemed suitable for meta-analysis because of the diversity in outcome measures and because it cannot be excluded that some patient data are used in multiple studies. The included studies have a prospective or retrospective character. Hence, in a number of studies, the data were collected primarily for clinical, not comparative, purposes. This review compares nTMS mapping with DCS mapping; in most included articles, this concerned intraoperative DCS mapping, but in the articles on epilepsy patients, an operatively placed subdural grid was used for DCS extraoperative language mapping. Both DCS techniques are not fully comparable. In this systematic review, the effect of nTMS mapping on treatment outcome was not evaluated.
Conclusion
nTMS mapping is a relatively new mapping technique for cortical function localization and can be a helpful and informative preoperative diagnostic tool. The largest body of evidence is available for nTMS motor mapping, in which the accuracy compared to DCS mapping is good. Concerning nTMS language mapping, there is more variability in accuracy results. The technique cannot replace intraoperative language mapping and should be used as an adjunct. The NPV and sensitivity of nTMS language mapping seem to be the most reliable, when nTMS is compared with DCS, especially in the anterior language areas. For now, only for nTMS motor and language mapping, comparative data with DCS are available. For other neurological functions, no comparative literature between both techniques is available yet. Further work should emphasize on the validation of nTMS mapping for other neurological functions, as well as other language tasks.
References
Aonuma S, Gomez-Tames J, Laakso I, Hirata A, Takakura T, Tamura M, Muragaki Y (2018) A high-resolution computational localization method for transcranial magnetic stimulation mapping. Neuroimage 172:85–93. https://doi.org/10.1016/j.neuroimage.2018.01.039
Babajani-Feremi A, Narayana S, Rezaie R, Choudhri AF, Fulton SP, Boop FA, Wheless JW, Papanicolaou AC (2015) Language mapping using high gamma electrocorticography, fMRI, and TMS versus electrocortical stimulation. Clin Neurophysiol 127:1822–1836. https://doi.org/10.1016/j.clinph.2015.11.017
Coburger J, Musahl C, Henkes H, Horvath-Rizea D, Bittl M, Weissbach C, Hopf N (2013) Comparison of navigated transcranial magnetic stimulation and functional magnetic resonance imaging for preoperative mapping in rolandic tumor surgery. Neurosurg Rev 36:65–75
Conti A, Raffa G, Granata F, Rizzo V, Germanò A, Tomasello F (2014) Navigated transcranial magnetic stimulation for “somatotopic” tractography of the cortico-spinal tract. Neurosurgery 10. https://doi.org/10.1227/NEU.0000000000000502
Diehl CD, Schwendner MJ, Sollmann N, Oechsner M, Meyer B, Combs SE, Krieg SM (2019) Application of presurgical navigated transcranial magnetic stimulation motor mapping for adjuvant radiotherapy planning in patients with high-grade gliomas. Radiother Oncol 138:30–37. https://doi.org/10.1016/j.radonc.2019.04.029
Finke M, Fadini T, Kantelhardt S, Giese A, Matthaus L, Schweikard A (2008) Brain-mapping using robotized TMS. Conf Proc IEEE Eng Med Biol Soc 2008:3929–3932
Forster M-T, Hattingen E, Senft C, Gasser T, Seifert V, Szelényi A (2011) Navigated transcranial magnetic stimulation and functional magnetic resonance imaging: advanced adjuncts in preoperative planning for central region tumors. Neurosurgery 68:1317–1324
Forster M-T, Heindl N, Hattingen E, Gessler F, Quick J, Seifert V, Senft C (2015) Brain surface reformatted imaging (BSRI) for intraoperative neuronavigation in brain tumor surgery. Acta Neurochir 157:265–274
Frey D, Strack V, Wiener E, Jussen D, Vajkoczy P, Picht T (2012) A new approach for corticospinal tract reconstruction based on navigated transcranial stimulation and standardized fractional anisotropy values. Neuroimage 62:1600–1609. https://doi.org/10.1016/j.neuroimage.2012.05.059
Fujiwara N, Sakatani K, Katayama Y, Murata Y, Hoshino T, Fukaya C, Yamamoto T (2004) Evoked-cerebral blood oxygenation changes in false-negative activations in BOLD contrast functional MRI of patients with brain tumors. Neuroimage 21:1464–1471. https://doi.org/10.1016/j.neuroimage.2003.10.042
Giglhuber K, Maurer S, Zimmer C, Meyer B, Krieg SM (2017) Evoking visual neglect-like deficits in healthy volunteers – an investigation by repetitive navigated transcranial magnetic stimulation. Brain Imaging Behav 11:17–29. https://doi.org/10.1007/s11682-016-9506-9
Giglhuber K, Maurer S, Zimmer C, Meyer B, Krieg SM (2018) Mapping visuospatial attention: the greyscales task in combination with repetitive navigated transcranial magnetic stimulation. BMC Neurosci 19:19. https://doi.org/10.1186/s12868-018-0440-1
Giussani C, Roux FE, Ojemann J, Pietro SE, Pirillo D, Papagno C (2010) Is preoperative functional magnetic resonance imaging reliable for language areas mapping in brain tumor surgery? Review of language functional magnetic resonance imaging and direct cortical stimulation correlation studies. Neurosurgery 66:113–120
Ille S, Drummer K, Giglhuber K, Conway N, Maurer S, Meyer B, Krieg SM (2018) Mapping of arithmetic processing by navigated repetitive transcranial magnetic stimulation in patients with parietal brain tumors and correlation with postoperative outcome. World Neurosurg 114:e1016–e1030. https://doi.org/10.1016/j.wneu.2018.03.136
Ille S, Engel L, Albers L, Schroeder A, Kelm A, Meyer B, Krieg SM (2019) Functional reorganization of cortical language function in glioma patients—a preliminary study. Front Oncol 9. https://doi.org/10.3389/fonc.2019.00446
Ille S, Picht T, Shiban E, Meyer B, Vajkoczy P, Krieg SM (2018) The impact of nTMS mapping on treatment of brain AVMs. Acta Neurochir 160:567–578. https://doi.org/10.1007/s00701-018-3475-2
Ille S, Sollmann N, Butenschoen VM, Meyer B, Ringel F, Krieg SM (2016) Resection of highly language-eloquent brain lesions based purely on rTMS language mapping without awake surgery. Acta Neurochir 158:2265–2275. https://doi.org/10.1007/s00701-016-2968-0
Ille S, Sollmann N, Hauck T, Maurer S, Tanigawa N, Obermueller T, Negwer C, Droese D, Boeckh-Behrens T, Meyer B, Ringel F, Krieg SM (2015) Impairment of preoperative language mapping by lesion location: a functional magnetic resonance imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation study. J Neurosurg 123:314–324
Ille S, Sollmann N, Hauck T, Maurer S, Tanigawa N, Obermueller T, Negwer C, Droese D, Zimmer C, Meyer B, Ringel F, Krieg SM (2015) Combined noninvasive language mapping by navigated transcranial magnetic stimulation and functional MRI and its comparison with direct cortical stimulation. J Neurosurg 123:212–225
Jung J, Lavrador JP, Patel S, Giamouriadis A, Lam J, Bhangoo R, Ashkan K, Vergani F (2019) First United Kingdom experience of navigated transcranial magnetic stimulation in preoperative mapping of brain tumors. World Neurosurg 122:e1578–e1587. https://doi.org/10.1016/j.wneu.2018.11.114
Kantelhardt SR, Fadini T, Finke M, Kallenberg K, Siemerkus J, Bockermann V, Matthaeus L, Paulus W, Schweikard A, Rohde V, Giese A (2010) Robot-assisted image-guided transcranial magnetic stimulation for somatotopic mapping of the motor cortex: a clinical pilot study. Acta Neurochir 152:333–343
Köhlert K, Jähne K, Saur D, Meixensberger J (2019) Neurophysiological examination combined with functional intraoperative navigation using TMS in patients with brain tumor near the central region—a pilot study. Acta Neurochir 161:1853–1864. https://doi.org/10.1007/s00701-019-04004-1
Krieg SM, Lioumis P, Mäkelä JP, Wilenius J, Karhu J, Hannula H, Savolainen P, Lucas CW, Seidel K, Laakso A, Islam M, Vaalto S, Lehtinen H, Vitikainen AM, Tarapore PE, Picht T (2017) Protocol for motor and language mapping by navigated TMS in patients and healthy volunteers; workshop report. Acta Neurochir 159:1187–1195
Krieg SM, Ringel F, Meyer B (2012) Functional guidance in intracranial tumor surgery. Perspect Med 1–12:59–64
Krieg SM, Shiban E, Buchmann N, Gempt J, Foerschler A, Meyer B, Ringel F (2012) Utility of presurgical navigated transcranial magnetic brain stimulation for the resection of tumors in eloquent motor areas: clinical article. J Neurosurg 116:994–1001. https://doi.org/10.3171/2011.12.JNS111524
Krieg SM, Shiban E, Buchmann N, Meyer B, Ringel F (2013) Presurgical navigated transcranial magnetic brain stimulation for recurrent gliomas in motor eloquent areas. Clin Neurophysiol 124:522–527
Krieg SM, Sollmann N, Hauck T, Ille S, Meyer B, Ringel F (2014) Repeated mapping of cortical language sites by preoperative navigated transcranial magnetic stimulation compared to repeated intraoperative DCS mapping in awake craniotomy. BMC Neurosci:15
Krieg SM, Tarapore PE, Picht T, Tanigawa N, Houde J, Sollmann N, Meyer B, Vajkoczy P, Berger MS, Ringel F, Nagarajan S (2014) Optimal timing of pulse onset for language mapping with navigated repetitive transcranial magnetic stimulation. Neuroimage 100:219–236
Krings T, Buchbinder BR, Butler WE, Chiappa KH, Jiang HJ, Rosen BR, Cosgrove GR (1997) Stereotactic transcranial magnetic stimulation: correlation with direct electrical cortical stimulation. Neurosurgery 41:1319–1326
Lehtinen H, Mäkelä JP, Mäkelä T, Lioumis P, Metsähonkala L, Hokkanen L, Wilenius J, Gaily E (2018) Language mapping with navigated transcranial magnetic stimulation in pediatric and adult patients undergoing epilepsy surgery: comparison with extraoperative direct cortical stimulation. Epilepsia Open 3:224–235. https://doi.org/10.1002/epi4.12110
Mangraviti A, Casali C, Cordella R, Legnani FG, Mattei L, Prada F, Saladino A, Contarino VE, Perin A, DiMeco F (2014) Practical assessment of preoperative functional mapping techniques: navigated transcranial magnetic stimulation and functional magnetic resonance imaging (vol 34, pg 1551, 2013). Neurol Sci 35:501. https://doi.org/10.1007/s10072-013-1430-9
Maurer S, Tanigawa N, Sollmann N, Hauck T, Ille S, Boeckh-Behrens T, Meyer B, Krieg SM (2015) Non-invasive mapping of calculation function by repetitive navigated transcranial magnetic stimulation. Brain Struct Funct 221:3927–3947. https://doi.org/10.1007/s00429-015-1136-2
Murad MH, Sultan S, Haffar S, Bazerbachi F (2018) Methodological quality and synthesis of case series and case reports. Evid Based Med 23:60–63. https://doi.org/10.1136/bmjebm-2017-110853
Negwer C, Ille S, Hauck T, Sollmann N, Maurer S, Kirschke JS, Ringel F, Meyer B, Krieg SM (2016) Visualization of subcortical language pathways by diffusion tensor imaging fiber tracking based on rTMS language mapping. Brain Imaging Behav 11:899–914. https://doi.org/10.1007/s11682-016-9563-0
Negwer C, Sollmann N, Ille S, Hauck T, Maurer S, Kirschke JS, Ringel F, Meyer B, Krieg SM (2016) Language pathway tracking: comparing nTMS-based DTI fiber tracking with a cubic ROIs-based protocol. J Neurosurg:1–9. https://doi.org/10.3171/2016.2.JNS152382
Opitz A, Zafar N, Bockermann V, Rohde V, Paulus W (2014) Validating computationally predicted TMS stimulation areas using direct electrical stimulation in patients with brain tumors near precentral regions. NeuroImage Clin 4:500–507
Ottenhausen M, Krieg SM, Meyer B, Ringel F (2015) Functional preoperative and intraoperative mapping and monitoring: increasing safety and efficacy in glioma surgery. Neurosurg Focus 38:E3. https://doi.org/10.3171/2014.10.FOCUS14611
Paiva WS, Fonoff ET, Marcolin MA, Bor-Seng-Shu E, Figueiredo EG, Teixeira MJ (2013) Navigated transcranial magnetic stimulation in preoperative planning for the treatment of motor area cavernous angiomas. Neuropsychiatr Dis Treat 9:1885–1888
Paiva WS, Fonoff ET, Marcolin MA, Cabrera HN, Teixeira MJ (2012) Cortical mapping with navigated transcranial magnetic stimulation in low-grade glioma surgery. Neuropsychiatr Dis Treat 8:197–201
Picht T, Krieg SM, Sollmann N, Rösler J, Niraula B, Neuvonen T, Savolainen P, Lioumis P, Mäkelä JP, Deletis V, Meyer B, Vajkoczy P, Ringel F (2013) A comparison of language mapping by preoperative navigated transcranial magnetic stimulation and direct cortical stimulation during awake surgery. Neurosurgery 72:808–819
Picht T, Mularski S, Kuehn B, Vajkoczy P, Kombos T, Suess O (2009) Navigated transcranial magnetic stimulation for preoperative functional diagnostics in brain tumor surgery. Neurosurgery 65:ons93–ons98
Picht T, Schilt S, Frey D, Vajkoczy P, Kufeld M (2014) Integration of navigated brain stimulation data into radiosurgical planning: potential benefits and dangers. Acta Neurochir 156:1125–1133. https://doi.org/10.1007/s00701-014-2079-8
Picht T, Schmidt S, Brandt S, Frey D, Hannula H, Neuvonen T, Karhu J, Vajkoczy P, Suess O (2011) Preoperative functional mapping for rolandic brain tumor surgery: comparison of navigated transcranial magnetic stimulation to direct cortical stimulation. Neurosurgery 69:581–588
Raffa G, Picht T, Scibilia A, Rösler J, Rein J, Conti A, Ricciardo G, Cardali SM, Vajkoczy P, Germanò A (2019) Surgical treatment of meningiomas located in the rolandic area: the role of navigated transcranial magnetic stimulation for preoperative planning, surgical strategy, and prediction of arachnoidal cleavage and motor outcome. J Neurosurg:1–12. https://doi.org/10.3171/2019.3.jns183411
Raffa G, Scibilia A, Conti A, Cardali SM, Rizzo V, Terranova C, Quattropani MC, Marzano G, Ricciardo G, Vinci SL, Germanò A (2019) Multimodal surgical treatment of high-grade gliomas in the motor area: the impact of the combination of navigated transcranial magnetic stimulation and fluorescein-guided resection. World Neurosurg 128:e378–e390. https://doi.org/10.1016/j.wneu.2019.04.158
Raffa G, Scibilia A, Conti A, Ricciardo G, Rizzo V, Morelli A, Angileri FF, Cardali SM, Germanò A (2019) The role of navigated transcranial magnetic stimulation for surgery of motor-eloquent brain tumors: a systematic review and meta-analysis. Clin Neurol Neurosurg 180:7–17
Rofes A, Mandonnet E, de Aguiar V, Rapp B, Tsapkini K, Miceli G (2019) Language processing from the perspective of electrical stimulation mapping. Cogn Neuropsychol 36:117–139
Säisänen L, Julkunen P, Kemppainen S, Danner N, Immonen A, Mervaala E, Määttä S, Muraja-Murro A, Könönen M, Schulder M, Anderson WS, Saisanen L, Julkunen P, Kemppainen S, Danner N, Immonen A, Mervaala E, Maatta S, Muraja-Murro A, Kononen M (2015) Locating and outlining the cortical motor representation areas of facial muscles with navigated transcranial magnetic stimulation. Neurosurgery 77:394–405. https://doi.org/10.1227/NEU.0000000000000798
Salminen-Vaparanta N, Noreika V, Revonsuo A, Koivisto M, Vanni S (2012) Is selective primary visual cortex stimulation achievable with TMS? Hum Brain Mapp 33:652–665. https://doi.org/10.1002/hbm.21237
Schwendner MJ, Sollmann N, Diehl CD, Oechsner M, Meyer B, Krieg SM, Combs SE (2018) The role of navigated transcranial magnetic stimulation motor mapping in adjuvant radiotherapy planning in patients with supratentorial brain metastases. Front Oncol 8:8. https://doi.org/10.3389/fonc.2018.00424
Seynaeve L, Haeck T, Gramer M, Maes F, De Vleeschouwer S, Van Paesschen W (2019) Optimized preoperative motor cortex mapping in brain tumors using advanced processing of transcranial magnetic stimulation data. NeuroImage Clin 21:101657. https://doi.org/10.1016/j.nicl.2019.101657
Sollmann N, Ille S, Negwer C, Boeckh-Behrens T, Ringel F, Meyer B, Krieg SM (2016) Cortical time course of object naming investigated by repetitive navigated transcranial magnetic stimulation. Brain Imaging Behav:1–15. https://doi.org/10.1007/s11682-016-9574-x
Takahashi S, Vajkoczy P, Picht T (2013) Navigated transcranial magnetic stimulation for mapping the motor cortex in patients with rolandic brain tumors. Neurosurg Focus 34:E3
Takakura T, Muragaki Y, Tamura M, Maruyama T, Nitta M, Niki C, Kawamata T (2017) Navigated transcranial magnetic stimulation for glioma removal: prognostic value in motor function recovery from postsurgical neurological deficits. J Neurosurg 127:877–891. https://doi.org/10.3171/2016.8.JNS16442
Tarapore PE, Findlay AM, Honma SM, Mizuiri D, Houde JF, Berger MS, Nagarajan SS (2013) Language mapping with navigated repetitive TMS: proof of technique and validation. Neuroimage 82:260–272
Tarapore PE, Tate MC, Honma SM, Mizuiri D, Berger MS, Nagarajan SS, Findlay AM, Honma SM, Mizuiri D, Berger MS, Nagarajan SS (2012) Preoperative multimodal motor mapping: a comparison of magnetoencephalography imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation: clinical article. J Neurosurg 117:354–362
Tokarev AS, Rak VA, Sinkin MV, Evdokimova OL, Stepanov VN, Koynash GV, Krieg SM, Krylov VV (2019) Appliance of navigated transcranial magnetic stimulation in radiosurgery for brain metastases. J Clin Neurophysiol 37:1–55. https://doi.org/10.1097/wnp.0000000000000621
Vitikainen A-M, Lioumis P, Paetau R, Salli E, Komssi S, Metsähonkala L, Paetau A, Kičić D, Blomstedt G, Valanne L, Mäkelä JP, Gaily E (2009) Combined use of non-invasive techniques for improved functional localization for a selected group of epilepsy surgery candidates. Neuroimage 45:342–348
Vitikainen A-M, Salli E, Lioumis P, Mäkelä JP, Metsähonkala L (2013) Applicability of nTMS in locating the motor cortical representation areas in patients with epilepsy. Acta Neurochir 155:507–518
Weiss C, Tursunova I, Neuschmelting V, Lockau H, Nettekoven C, Oros-Peusquens A-M, Stoffels G, Rehme AK, Faymonville AM, Shah NJ, Langen KJ, Goldbrunner R, Grefkes C (2015) Improved nTMS- and DTI-derived CST tractography through anatomical ROI seeding on anterior pontine level compared to internal capsule. NEUROIMAGE-CLINICAL 7:424–437. https://doi.org/10.1016/j.nicl.2015.01.006
Acknowledgments
The authors thank Sjoukje van der Werf, librarian at the medical library of the University Medical Center Groningen, for helping to design and perform the search strategies for the different electronic databases.
Funding
Open Access funding provided by University Medical Center Groningen (UMCG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. R.B. is partially supported within the framework of a subsidy by the Russian Academic Excellence Project ‘5-100’.
Ethical approval
For this type of paper, no approval of the local ethical committee was deemed necessary. The work is in accordance with the declaration of Helsinki and its later amendments, for as far applicable.
Informed consent
Not applicable, since the systematic review contains no new patient data/inclusion.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplement 1
Search strings used in the three different electronic databases (DOCX 13 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jeltema, HR., Ohlerth, AK., de Wit, A. et al. Comparing navigated transcranial magnetic stimulation mapping and “gold standard” direct cortical stimulation mapping in neurosurgery: a systematic review. Neurosurg Rev 44, 1903–1920 (2021). https://doi.org/10.1007/s10143-020-01397-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-020-01397-x