Abstract
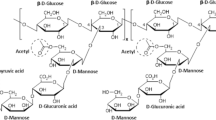
In this work, the production and downstream processing of gellan gum obtained from milk skin residue (MSR) as a carbon source was investigated. The different medium variables, namely shaking speed, inoculum size, and nitrogen source, on gellan gum production were studied. The suitable medium variables were found to be shaking speed (150 rpm); inoculum size (5% v/v), and nitrogen source (yeast extract, YE). The production of gellan gum was compared for various carbon sources, namely fructose, sucrose, glucose, lactose, and MSR. In order to obtain higher gellan gum concentration, response surface methodology was employed for optimizing MSR to glucose ratio, initial pH, and yeast extract concentration. The maximum gellan gum concentration of 8.98 ± 0.42 g/L was obtained at the following optimum conditions: ratio of MSR and glucose = 1:0.743, initial pH (7.5), and yeast extract concentration 8 g/L. The concentration of gellan gum was increased by 2.73-fold than un-optimized medium. The various factors influencing the recovery of gellan gum, namely screening of solvents, ratio of solvent to supernatant (v/v), and incubation time for precipitation, were also studied. The purified gellan gum was characterized by thermogravimetric analysis (TGA/DSC), Fourier transform infrared spectroscopy, scanning electron microscope, and X-ray diffraction analysis.





Similar content being viewed by others
References
Schmid J, Sieber V, Rehm B (2015) Bacterial exopolysaccharides: biosynthesis pathways and engineering strategies. Front Microbiol 6:1–24
Xu X, Nie Z, Zheng Z, Zhu L, Zhan X (2017) Production and rheological properties of welan gum produced by Sphingomonas sp. ATCC 31555 with different nitrogen sources. J Mol Microbiol Biotechnol 27:55–63
Thomas DJ, Jessop ZM, Whitaker IS (2017) 3D bioprinting for reconstructive surgery: techniques and applications
Fialho AM, Martins LO, Donval ML et al (1999) Structures and properties of gellan polymers produced by Sphingomonas paucimobilis ATCC 31461 from lactose compared with those produced from glucose and from cheese whey. Appl Environ Microbiol 65:2485–2491
West TP, Strohfus B (1998) Effect of complex nitrogen sources upon gellan production by Sphingomonas paucimobilis ATCC 31461. Microbios 94:145–152
Bajaj IB, Survase SA, Saudagar PS, Singhal RS (2007) Gellan gum: fermentative production, downstream processing and applications. Food Technol Biotechnol 45:341–354
Di Donato P, Finore I, Anzelmo G et al (2014) Biomass and biopolymer production using vegetable wastes as cheap substrates for extremophiles. Chem Eng Trans 38:163–168
Huang J, Zhu S, Li C, Zhang C, Ji Y (2020) Cost-effective optimization of gellan gum production by Sphingomonas paucimobilis using corn steep liquor. Prep Biochem Biotechnol 50:191–197
Liu X, Lin L, Xu X, Zhang H, Wu L, Zhu P, Li S, Xu H (2018) Two-step economical welan gum production by Sphingomonas sp. HT-1 from sugar industrial by-products. Carbohydr Polym 181:412–418
Raghunandan K, Kumar A, Kumar S et al (2018) Production of gellan gum, an exopolysaccharide, from biodiesel-derived waste glycerol by Sphingomonas spp. 3 Biotech 8:1–13. https://doi.org/10.1007/s13205-018-1096-3
Monteiro GA, Fialho AM, Ripley SJ, Sá-Correia I (1992) Electrotransformation of gellan-gum producing and non-producing Pseudomonas elodea strains. J Appl Bacteriol 72:423–428
Huang J, Jiang S, Xu X, Wu H, Zhu X, Ke Z, Cai J, Huang L, Xu Z (2012) Effects of carbon/nitrogen ratio, dissolved oxygen and impeller type on gellan gum production in Sphingomonas paucimobilis. Ann Microbiol 62:299–305
Zhang J, Dong YC, Fan LL, Jiao ZH, Chen QH (2015) Optimization of culture medium compositions for gellan gum production by a halobacterium Sphingomonas paucimobilis. Carbohydr Polym 115:694–700
Bajaj IB, Saudagar PS, Singhal RS, Pandey A (2006) Statistical approach to optimization of fermentative production of gellan gum from Sphingomonas paucimobilis ATCC 31461. J Biosci Bioeng 102:150–156
Wang X, Xu P, Yuan Y et al (2006) Modeling for gellan gum production by Sphingomonas paucimobilis ATCC 31461 in a simplified medium. Microbiology 72:3367–3374
Banik RM, Santhiagu A, Upadhyay SN (2007) Optimization of nutrients for gellan gum production by Sphingomonas paucimobilis ATCC-31461 in molasses based medium using response surface methodology. Bioresour Technol 98:792–797
Freitas F, Alves VD, Pais J, Costa N, Oliveira C, Mafra L, Hilliou L, Oliveira R, Reis MAM (2009) Characterization of an extracellular polysaccharide produced by a Pseudomonas strain grown on glycerol. Bioresour Technol 100:859–865
Wang D, Kim H, Lee S, Kim DH, Joe MH (2020) Improved gellan gum production by a newly-isolated Sphingomonas azotifigens GL-1 in a cheese whey and molasses based medium. Process Biochem 95:269–278
El-Shora NM, Metwally MAA (2008) Production, purification and characterisation of proteases from whey by some fungi. Ann Microbiol 58:495–502
Ryan MP, Walsh G (2016) The biotechnological potential of whey. Rev Environ Sci Biotechnol 15:479–498
Zohri A-NA (2000) Glycerol production from cheese whey by selected fungal cultures. J Food Sci Technol 37(5):533–538
Guimarães PMR, Teixeira JA, Domingues L (2010) Fermentation of lactose to bio-ethanol by yeasts as part of integrated solutions for the valorisation of cheese whey. Biotechnol Adv 28:375–384
Qureshi N, Friedl A, Maddox IS (2014) Butanol production from concentrated lactose/whey permeate: use of pervaporation membrane to recover and concentrate product. Appl Microbiol Biotechnol 98:9859–9867
Choudhury AR, Sharma N, Prasad GS (2012) Deoiledjatropha seed cake is a useful nutrient for pullulan production. Microb Cell Fact 11:3–11
Feeley RM, Criner PE, HTS (1975) Major fatty acids and proximate composition of dairy products. J Am Diet Assoc 66:140–146
Li H, Xu H, Xu H, Li S, Ying HJ, Ouyang PK (2011) Enhanced welan gum production using a two-stage agitation speed control strategy in Alcaligenes sp. CGMCC2428. Bioprocess Biosyst Eng 34:95–102
Singh RS, Saini GK, Kennedy JF (2009) Downstream processing and characterization of pullulan from a novel colour variant strain of Aureobasidium pullulans FB-1. Carbohydr Polym 78:89–94
Göksungur Y, Dǎgbaǧli S, Uçan A, Güvenç U (2005) Optimization of pullulan production from synthetic medium by Aureobasidium pullulans in a stirred tank reactor by response surface methodology. J Chem Technol Biotechnol 80:819–827
Giavasis I, Harvey LM, McNeil B (2006) The effect of agitation and aeration on the synthesis and molecular weight of gellan in batch cultures of Sphingomonas paucimobilis. Enzyme Microb Technol 38:101–108
Schilling BM, Rau U, Maier T, Fankhauser P (1999) Modeling and scale-up of the unsterile scleroglucan production process with Sclerotium rolfsii ATCC 15205. Bioprocess Eng 20:195–201
Gao H, Chen J, Du G et al (2003) Effect of agitation and mixing on hyaluronic acid production by Streptococcus zooepidemicus. J Chem Ind Eng 54:350–356
Kim JH, Yoo SJ, Oh DK, Kweon YG, Park DW, Lee CH, Gil GH (1996) Selection of a Streptococcus equi mutant and optimization of culture conditions for the production of high molecular weight hyaluronic acid. Enzyme Microb Technol 19:440–445
Kanari B, Banik RR, Upadhyay SN (2002) Effect of environmental factors and carbohydrate on gellan gum production. Appl Biochem Biotechnol - Part A Enzym Eng Biotechnol 102–103:129–140
Hamieh A, Olama Z, Holail H (2013) Microbial production of polyhydroxybutyrate , a biodegradable plastic using agro-industrial waste. Glob Adv Res J Microbiol 2:54–64
Cheng Z, Yang R, Liu X, Liu X, Chen H (2017) Green synthesis of bacterial cellulose via acetic acid pre-hydrolysis liquor of agricultural corn stalk used as carbon source. Bioresour Technol 234:8–14
Lazaridou A, Roukas T, Biliaderis CG, Vaikousi H (2002) Characterization of pullulan produced from beet molasses by Aureobasidium pullulans in a stirred tank reactor under varying agitation. Enzyme Microb Technol 31:122–132
Xu X-Y, Dong S-H, Li S, Chen XY, Wu D, Xu H (2015) Statistical experimental design optimization of rhamsan gum production by Sphingomonas sp. CGMCC 6833. J Microbiol 53:272–278
Bechem EET (2012) Utilisation of organic and inorganic nitrogen sources by Scleroderma sinnamariense Mont. isolated from Gnetum africanum Welw. African J Biotechnol 11:9205–9213
Dreveton E, Monot F, Lecourtier J, Ballerini D, Choplin L (1996) Influence of fermentation hydrodynamics on gellan gum physico-chemical characteristics. J Ferment Bioeng 82:272–276
Faria S, De Oliveira Petkowicz CL, De Morais SAL et al (2011) Characterization of xanthan gum produced from sugar cane broth. Carbohydr Polym 86:469–476
Prajapati VD, Jani GK, Zala BS, Khutliwala TA (2013) An insight into the emerging exopolysaccharide gellan gum as a novel polymer. Carbohydr Polym 93:670–678
Gaur R, Singh R, Tiwari S, Yadav SK, Daramwal NS (2010) Optimization of physico-chemical and nutritional parameters for a novel pullulan-producing fungus, Eurotium chevalieri. J Appl Microbiol 109:1035–1043
Arockiasamy S, Banik RM (2008) Optimization of gellan gum production by Sphingomonas paucimobilis ATCC 31461 with nonionic surfactants using central composite design. J Biosci Bioeng 105:204–210
Nampoothiri KM, Singhania RR, Sabarinath C, Pandey A (2003) Fermentative production of gellan using Sphingomonas paucimobilis. Process Biochem 38:1513–1519
Zhu G, Sheng L, Tong Q (2013) A new strategy to enhance gellan production by two-stage culture in Sphingomonas paucimobilis. Carbohydr Polym 98:829–834
Heilig ML (1994) United States Patent Office. ACM SIGGRAPH Comput Graph 28:131–134
Ying XX, Ping Z, Sha L, Ye CX, Zhong Y, Hong X (2014) Production of rhamsan gum using a two-stage pH control strategy by Sphingomonas sp. CGMCC 6833. Appl Biochem Biotechnol 172:168–175
Ai H, Liu M, Yu P, Zhang S, Suo Y, Luo P, Li S, Wang J (2015) Improved welan gum production by Alcaligenes sp. ATCC31555 from pretreated cane molasses. Carbohydr Polym 129:35–43
Hamidi M, Kennedy JF, Khodaiyan F, Mousavi Z, Hosseini SS (2019) Production optimization, characterization and gene expression of pullulan from a new strain of Aureobasidium pullulans. Int J Biol Macromol 138:725–735
Wang D, Ju X, Zhou D, Wei G (2014) Efficient production of pullulan using rice hull hydrolysate by adaptive laboratory evolution of Aureobasidium pullulans. Bioresour Technol 164:12–19
Nikonenko NA, Buslov DK, Sushko NI, RGZ (2000) Investigation of stretching vibrations of glycosidic linkages in disaccharides and polysaccharides with use of IR spectra deconvolution. Biopolym Orig Res Biomol 57:257–262
Karthika JS, Vishalakshi B (2015) Novel stimuli responsive gellan gum-graft-poly(DMAEMA) hydrogel as adsorbent for anionic dye. Int J Biol Macromol 81:648–655
Arun Krishna K, Vishalakshi B (2017) Gellan gum-based novel composite hydrogel: Evaluation as adsorbent for cationic dyes. J Appl Polym Sci 134:1–9
Basta AH, El-Saied H (2009) Performance of improved bacterial cellulose application in the production of functional paper. J Appl Microbiol 107:2098–2107
Rosca I, Petrovici AR, Peptanariu D, Nicolescu A, Dodi G, Avadanei M, Ivanov IC, Bostanaru AC, Mares M, Ciolacu D (2018) Biosynthesis of dextran by Weissella confusa and its In vitro functional characteristics. Int J Biol Macromol 107:1765–1772
Dixit R, Verma A, Singh UP et al (2011) Preparation and characterization of gellan-chitosan polyelectrolyte complex beads. Lat Am J Pharm 30:1186–1195
Yang F, Xia S, Tan C, Zhang X (2013) Preparation and evaluation of chitosan-calcium-gellan gum beads for controlled release of protein. Eur Food Res Technol 237:467–479
Mao R, Tang J, Swanson BG (2001) Water holding capacity and microstructure of gellan gels. Carbohydr Polym 46:365–371
Acknowledgments
All authors are thankful to the Management SASTRA Deemed to be University, Thanjavur, Tamil Nadu, India, for providing the necessary facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1427 kb)
Rights and permissions
About this article
Cite this article
Hari Suthan Viswanathan, Sameeha Syed Abdul Rahman, Ponnusami Venkatachalam et al. Production of gellan gum using milk skin residue (MSR)—a tea shop waste: statistical optimization and downstream processing. Biomass Conv. Bioref. 13, 189–203 (2023). https://doi.org/10.1007/s13399-020-01026-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01026-z