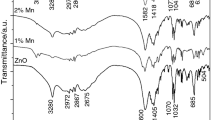
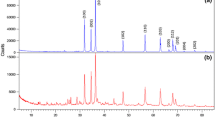
Abstract
Crystallization kinetics of metal oxides is an exciting subject because it directly relates to the structure and property of the metal oxides. Recently, doping ZnO by transition metals shown advantageous in the modification of the morphology of ZnO in photocatalytic applications as well as other specific applications. In the current research, Mn–Fe co-doped ZnO nano-powders synthesized via the Pechini sol–gel process. Through differential scanning calorimetry (DSC), X-ray diffraction (XRD), High-Resolution transmission electron microscopy (HR-TEM), field emission scanning electron microscopy (FE-SEM), and selected area electron diffraction (SAED) the crystallization kinetics, growth mechanism; morphological as well as the structural characteristics of the Mn–Fe co-doped ZnO dry gels studied, respectively. The XRD and SAED results revealed that the dry gels calcined at 800 °C for 2 h have a wurtzite symmetry. By a non-isothermal DSC technique, the crystallization activation energy for wurtzite Mn–Fe co-doped ZnO calculated ~308.19 kJ/mol. Bulk nucleation was dominant in the wurtzite Mn–Fe co-doped ZnO crystallization; since the crystallization growth mechanism index (m) and morphology parameter (n) were close to 3.0. Wurtzite Mn–Fe co-doped ZnO powders had a semispherical morphology with a primary particle size from 20 to 30 nm using the electron microscopy examination.

Highlights
-
Mn–Fe co-doped ZnO nanoparticles was synthesized with a simple Pechini sol–gel procedure.
-
Kinetic Data for Crystallization was carried out under non-isothermal condition using Johnson–Mehl–Avrami model.
-
Activation energy to crystallize the Mn–Fe co-doped ZnO nanopowder via Pechini sol–gel process was ~308.19 kJ mol−1.
-
crystallization mechanism index (m) and the growth morphology parameter (n) were calculated ~3.0.
-
Electron Microscopy results showed a spherical-like morphology and the particle sizes were roughly between 20 and 30 nm.











Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ong CB, Ng LY, Mohammad AW (2018) A review of ZnO nanoparticles as solar photocatalysts: synthesis, mechanisms and applications. Renew Sustain Energy Rev 81:536–551
Zhou C, Ghods A, Yunghans KL, Saravade VG, Patel PV, Jiang X, Kucukgok B, Lu N, Ferguson I (2017) ZnO for solar cell and thermoelectric applications. In: Oxide-based materials and devices VIII. International Society for Optics and Photonics, San Francisco, California, United States, p 101051
Keis K, Bauer C, Boschloo G, Hagfeldt A, Westermark K, Rensmo H, Siegbahn H (2002) Nanostructured ZnO electrodes for dye-sensitized solar cell applications. J Photochem Photobiol A: Chem 148(1-3):57–64
Huang J, Yin Z, Zheng Q (2011) Applications of ZnO in organic and hybrid solar cells. Energy Environ Sci 4(10):3861–3877
Tyona MD (2019) Doped zinc oxide nanostructures for photovoltaic solar cells application. In: Zinc oxide based nano materials and devices, Ahmed M. Nahhas, IntechOpen. https://doi.org/10.5772/intechopen.86254
Silambarasan M, Saravanan S, Ohtani N, Tetsuo S (2014) Structural and photoluminescence studies of Ni-doped ZnO nanoparticles synthesized by solution combustion method. MRS Online Proceedings Library Archive 1584, Cambridge University Press, England
Malapati V, Venkataratnam K, Singh R (2018) Structure and magnetic properties of Mn–Fe co-doped ZnO thin films deposited by RF-magnetron sputtering. In: AIP Conf Proc. vol 1. AIP Publishing LLC, p 080055
Ashraf R, Riaz S, Bashir M, Khan U, Naseem S (2014) Structural and magnetic properties of Mn/Fe co-doped ZnO thin films prepared by sol–gel technique. IEEE Trans Magn 50(8):1–4
Middha S, Nikita, Yadav A, Kumar D (2018) Synthesis, characterization, and properties of Mn2+ doped ZnO nanoparticles. In: AIP Conf Proc. vol 1. AIP Publishing LLC, p 020006
Sharma P, Gupta A, Rao K, Owens FJ, Sharma R, Ahuja R, Guillen JO, Johansson B, Gehring G (2003) Ferromagnetism above room temperature in bulk and transparent thin films of Mn-doped ZnO. Nat Mater 2(10):673–677
Cheng W, Ma X (2009) Structural, optical and magnetic properties of Fe-doped ZnO. In: J Phys Conf Ser p 012039
Wu X, Wei Z, Zhang L, Wang X, Yang H, Jiang J (2014) Optical and magnetic properties of Fe doped ZnO nanoparticles obtained by hydrothermal synthesis. J Nanomater
Singhal R, Fernando M, LeMaire PK, Wu B (2019) Characterization of ZnO and Fe doped ZnO nanoparticles using fluorescence spectroscopy. In: Oxide-based materials and devices X. International Society for Optics and Photonics, San Francisco, California, United States, p 109192
Simanjuntak FM, Prasad OK, Panda D, Lin C-A, Tsai T-L, Wei K-H, Tseng T-Y (2016) Impacts of Co doping on ZnO transparent switching memory device characteristics. Appl Phys Lett 108(18):183506
Cui J, Zeng Q, Gibson UJ (2006) Synthesis and magnetic properties of Co-doped ZnO nanowires. J Appl Phys 99(8):08M113
Ciciliati MA, Silva MF, Fernandes DM, de Melo MA, Hechenleitner AAW, Pineda EA (2015) Fe-doped ZnO nanoparticles: synthesis by a modified sol–gel method and characterization. Mater Lett 159:84–86
Levy D, Zayat M (2015) The sol–gel handbook, 3 Volume Set: Synthesis, characterization, and applications, vol 2. John Wiley & Sons, Germany
Sakka S, Kozuka H (2005) Handbook of sol–gel science and technology. 1. sol–gel processing, vol 1. Springer Science & Business Media, London, England
Çelikbilek M, Ersundu AE, Aydın S (2012) Crystallization kinetics of amorphous materials. Advances in crystallization processes, InTech, Croatia, 127–162
Malek J, Matsuda S, Watanabe A, Ikegami T, Mitsuhashi T (1995) Crystallization kinetics of zirconia-yttria gels. Thermochim Acta 267:181–194
Málek J (2000) Kinetic analysis of crystallization processes in amorphous materials. Thermochim Acta 355(1):239–253
Malek J (1999) Crystallization kinetics by thermal analysis. J Therm Anal Calorim 56(2):763–769
Stojanović B, Marinković Z, Branković G, Fidančevska E (2000) Evaluation of kinetic data for crystallization of TiO2 prepared by hydrolysis method. J Therm Anal Calorim 60(2):595–604
Henderson DW (1979) Experimental analysis of non-isothermal transformations involving nucleation and growth. J Therm Anal Calorim 15(2):325–331
Henderson DW (1979) Thermal analysis of non-isothermal crystallization kinetics in glass forming liquids. J Non-Crystalline Solids 30(3):301–315
Ismail A, Menazea A, Kabary HA, El-Sherbiny A, Samy A (2019) The influence of calcination temperature on structural and antimicrobial characteristics of zinc oxide nanoparticles synthesized by sol–gel method. J Mol Struct 1196:332–337
Birkholz M Principles of X‐ray diffraction (2006) Thin film analysis by X-ray scattering p 1–40
Srinivasan R, De Angelis RJ, Ice G, Davis BH (1991) Identification of tetragonal and cubic structures of zirconia using synchrotron x-radiation source. J Mater Res 6(06):1287–1292
Oghaz MH, Razavi RS, Loghman-Estark MR, Ghasemi R (2013) Optimization of morphology and particle size of modified sol gel synthesized YSZ nanopowder using Taguchi method. J Nano Res 21:65–70
Hajizadeh-Oghaz M, Razavi RS, Estarki ML (2014) Large-scale synthesis of YSZ nanopowder by Pechini method. Bull Mater Sci 37(5):969–973
Hajizadeh-Oghaz M, Razavi RS, Khajelakzay M Optimizing sol–gel synthesis of magnesia-stabilized zirconia (MSZ) nanoparticles using Taguchi robust design for thermal barrier coatings (TBCs) applications. J Sol–gel Sci Technol 73(1) p 1–15
Hajizadeh-Oghaz M, Razavi RS, Loghman-Estarki MR (2014) Synthesis and characterization of non-transformable tetragonal YSZ nanopowder by means of Pechini method for thermal barrier coatings (TBCs) applications. J Sol–gel Sci Technol 70(1):6–13
Ganesh RS, Durgadevi E, Navaneethan M, Patil V, Ponnusamy S, Muthamizhchelvan C, Kawasaki S, Patil P, Hayakawa Y (2017) Low temperature ammonia gas sensor based on Mn-doped ZnO nanoparticle decorated microspheres. J Alloy Compd 721:182–190
Hui A, Ma J, Liu J, Bao Y, Zhang J (2017) Morphological evolution of Fe doped sea urchin-shaped ZnO nanoparticles with enhanced photocatalytic activity. J Alloy Compd 696:639–647
Wu Y, Wang X (2011) Preparation and characterization of single-phase α-Fe2O3 nano-powders by Pechini sol–gel method. Mater Lett 65(13):2062–2065
da Conceicao L, Ribeiro NF, Souza MM (2011) Synthesis of La1−xSrxMnO3 powders by polymerizable complex method: evaluation of structural, morphological and electrical properties. Ceram Int 37(7):2229–2236
Motta M, Deimling C, Saeki MJ, Lisboa-Filho PN (2008) Chelating agent effects in the synthesis of mesoscopic-size superconducting particles. J Sol–gel Sci Technol 46(2):201–207
Kuo C-W, Lee Y-H, Hung I, Wang M-C, Wen S-B, Fung K-Z, Shih C-J (2008) Crystallization kinetics and growth mechanism of 8mol% yttria-stabilized zirconia (8YSZ) nano-powders prepared by a sol–gel process. J Alloy Compd 453(1):470–475
Acknowledgements
The Central Research Laboratory of the Esfarayen University of Technology would be highly appreciated for the assistance provided to conduct this study.
Author information
Authors and Affiliations
Contributions
Morteza Hajizadeh-Oghaz as the first and corresponding author developed all of the theoretical formalism, performed the analytic calculations, and performed the numerical simulations. He also contributed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent for publication
Hereby I transfer to Springer the non-exclusive publication rights and I warrant that my contribution is original and that I have full power to make this grant. The author signs for and accepts responsibility for releasing this material on behalf of all co-authors. This transfer of publication rights covers the non-exclusive right to reproduce and distribute the article, including reprints, translations, photographic reproductions, microform, electronic form (offline, online) or any other reproductions of similar nature.
Ethics approval and consent to participate
This paper does not contain any studies with human or animal participants performed by any of the authors. Informed consent was obtained from all individual participants included in the study.
Additional information
Publishers note
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hajizadeh-Oghaz, M. Evaluation of kinetic data for crystallization of Mn–Fe co-doped ZnO nanoparticles synthesized via sol–gel process. J Sol-Gel Sci Technol 96, 276–286 (2020). https://doi.org/10.1007/s10971-020-05373-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-020-05373-1