Abstract
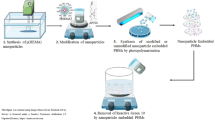
In this experimental study, a novel bionanocomposite of gelatin as a polypeptide hydrogel consisting of copper (II) oxide nanomaterial (GL–CuO) was prepared. After dispersing the synthesized CuO nanoparticles (NPs) within the GL aqueous solution, they were cross-linked by the addition of a formaldehyde solution. The resulting GL–CuO nanocomposite hydrogels were further characterized by FESEM, EDX, XRD, XPS, and FTIR. The FESEM micrographs confirmed the uniform distribution of CuO NPs in the hydrogel matrix, with dimensions ranging from 60 to 80 nm. The XRD patterns confirmed the presence of the monoclinic crystalline form of CuO NPs embedded in the GL hydrogel. The GL–CuO catalyst was tested in the reduction of two azo dyes. All the reactions were carried out in the presence of a strong reducing agent, NaBH4, with the catalyst demonstrating high activities toward methyl orange and congo red with reaction rate constants of 9.91 × 10−1 min−1 and 5.8 × 10−1 min−1, respectively. The reduction of these dyes was followed by UV–visible spectroscopy that confirmed complete reduction of the dye.

Highlights
-
A facile method for the preparation of polypeptide (gelatin) CuO hydrogel nanocomposite.
-
The catalytic properties of GL–CuO in the reduction of azo dyes have been studied.
-
The organic pollutant, MO, is reduced by hydrogel nanocomposite with a high rate constant.
-
During recyclability, the catalytic activity of hydrogel nanocomposite decreases.
-
The catalyst recovery is easy and requires only filtration.










Similar content being viewed by others
References
Ali F, Khan SB, Kamal T, Anwar Y, Alamry KA, Asiri AM (2017) Anti-bacterial chitosan/zinc phthalocyanine fibers supported metallic and bimetallic nanoparticles for the removal of organic pollutants. Carbohydr Polym 173:676–689. https://doi.org/10.1016/j.carbpol.2017.05.074
Khan SA, Khan SB, Kamal T, Yasir M, Asiri AM (2016) Antibacterial nanocomposites based on chitosan/Co-MCM as a selective and efficient adsorbent for organic dyes. Int J Biol Macromol 91:744–751. https://doi.org/10.1016/j.ijbiomac.2016.06.018
Ali F, Khan SB, Kamal T, Anwar Y, Alamry KA, Asiri AM (2017) Bactericidal and catalytic performance of green nanocomposite based-on chitosan/carbon black fiber supported monometallic and bimetallic nanoparticles. Chemosphere 188:588–598. https://doi.org/10.1016/j.chemosphere.2017.08.118
Khan SB, Ali F, Kamal T, Anwar Y, Asiri AM, Seo J (2016) CuO embedded chitosan spheres as antibacterial adsorbent for dyes. Int J Biol Macromol 88:113–119. https://doi.org/10.1016/j.ijbiomac.2016.03.026
Kamal T (2017) High performance NiO decorated graphene as a potential H2 gas sensor. J Alloys Compd 729:1058–1063. https://doi.org/10.1016/j.jallcom.2017.09.124
Kavitha T, Haider S, Kamal T, Ul-Islam M (2017) Thermal decomposition of metal complex precursor as route to the synthesis of Co3O4 nanoparticles: antibacterial activity and mechanism. J Alloys Compd 704:296–302. https://doi.org/10.1016/j.jallcom.2017.01.306
Ahmad I, Khan SB, Kamal T, Asiri AM (2017) Visible light activated degradation of organic pollutants using zinc–iron selenide. J Mol Liq 229:429–435. https://doi.org/10.1016/j.molliq.2016.12.061
Kavitha T, Kumar S, Prasad V, Asiri AM, Kamal T, Ul-Islam M (2019) NiO powder synthesized through nickel metal complex degradation for water treatment. Desalination Water Treat 155:216–224. https://doi.org/10.5004/dwt.2019.24054
Ahmed MS, Kamal T, Khan SA, Anwar Y, Saeed MT, Asiri AM, Khan SB (2016) Assessment of anti-bacterial Ni-Al/chitosan composite spheres for adsorption assisted photo-degradation of organic pollutants. Curr Nanosci 12:569–575. https://doi.org/10.2174/1573413712666160204000517
Dhakshinamoorthy J, Pullithadathil B (2016) New insights towards electron transport mechanism of highly efficient p-type CuO (111) nanocuboids-based H2S gas sensor. J Phys Chem C 120:4087–4096. https://doi.org/10.1021/acs.jpcc.5b11327
Hasheminya S-M, Mokarram RR, Ghanbarzadeh B, Hamishekar H, Kafil HS, Dehghannya J (2019) Influence of simultaneous application of copper oxide nanoparticles and Satureja Khuzestanica essential oil on properties of kefiran-carboxymethyl cellulose films. Polym Test 73:377–388. https://doi.org/10.1016/j.polymertesting.2018.12.002
Haider A, Haider S, Kang I-K, Kumar A, Kummara MR, Kamal T, Han SS (2018) A novel use of cellulose based filter paper containing silver nanoparticles for its potential application as wound dressing agent. Int J Biol Macromol 108:455–461. https://doi.org/10.1016/j.ijbiomac.2017.12.022
Kamal T, Ul-Islam M, Khan SB, Asiri AM (2015) Adsorption and photocatalyst assisted dye removal and bactericidal performance of ZnO/chitosan coating layer. Int J Biol Macromol 81:584–590. https://doi.org/10.1016/j.ijbiomac.2015.08.060
Ahmad I, Kamal T, Khan SB, Asiri AM (2016) An efficient and easily retrievable dip catalyst based on silver nanoparticles/chitosan-coated cellulose filter paper. Cellulose 23:3577–3588. https://doi.org/10.1007/s10570-016-1053-4
Kamal T, Anwar Y, Khan SB, Chani MTS, Asiri AM (2016) Dye adsorption and bactericidal properties of TiO2/chitosan coating layer. Carbohydr Polym 148:153–160. https://doi.org/10.1016/j.carbpol.2016.04.042
Haider S, Kamal T, Khan SB, Omer M, Haider A, Khan FU, Asiri AM (2016) Natural polymers supported copper nanoparticles for pollutants degradation. Appl Surf Sci 387:1154–1161. https://doi.org/10.1016/j.apsusc.2016.06.133
Kamal T, Khan SB, Asiri AM (2016) Nickel nanoparticles-chitosan composite coated cellulose filter paper: an efficient and easily recoverable dip-catalyst for pollutants degradation. Environ Pollut 218:625–633. https://doi.org/10.1016/j.envpol.2016.07.046
Kamal T, Khan SB, Asiri AM (2016) Synthesis of zero-valent Cu nanoparticles in the chitosan coating layer on cellulose microfibers: evaluation of azo dyes catalytic reduction. Cellulose 23:1911–1923. https://doi.org/10.1007/s10570-016-0919-9
Kamal T, Khan SB, Haider S, Alghamdi YG, Asiri AM (2017) Thin layer chitosan-coated cellulose filter paper as substrate for immobilization of catalytic cobalt nanoparticles. Int J Biol Macromol 104:56–62. https://doi.org/10.1016/j.ijbiomac.2017.05.157
Ul-Islam M, Ullah MW, Khan S, Kamal T, Ul-Islam S, Shah N, Park JK (2016) Recent advancement in cellulose based nanocomposite for addressing environmental challenges. Recent Pat Nanotechnol 10:169–180. https://doi.org/10.2174/1872210510666160429144916
Kamal T, Ahmad I, Khan SB, Asiri AM (2019) Bacterial cellulose as support for biopolymer stabilized catalytic cobalt nanoparticles. Int J Biol Macromol 135:1162–1170. https://doi.org/10.1016/j.ijbiomac.2019.05.057
Kamal T, Ahmad I, Khan SB, Asiri AM (2017) Synthesis and catalytic properties of silver nanoparticles supported on porous cellulose acetate sheets and wet-spun fibers. Carbohydr Polym 157:294–302. https://doi.org/10.1016/j.carbpol.2016.09.078
Kamal T (2019) Aminophenols formation from nitrophenols using agar biopolymer hydrogel supported CuO nanoparticles catalyst. Polym Test 77:105896. https://doi.org/10.1016/j.polymertesting.2019.105896
Khan MSJ, Khan SB, Kamal T, Asiri AM (2019) Agarose biopolymer coating on polyurethane sponge as host for catalytic silver metal nanoparticles. Polym Test 78:105983. https://doi.org/10.1016/j.polymertesting.2019.105983
Kamal T, Ali N, Naseem AA, Khan SB, Asiri AM (2016) Polymer nanocomposite membranes for antifouling nanofiltration. Recent Pat Nanotechnol 10:189–201. https://doi.org/10.2174/1872210510666160429145704
Khan FU, Asimullah, Khan SB, Kamal T, Asiri AM, Khan IU, Akhtar K (2017) Novel combination of zero-valent Cu and Ag nanoparticles @ cellulose acetate nanocomposite for the reduction of 4-nitro phenol. Int J Biol Macromol 102:868–877. https://doi.org/10.1016/j.ijbiomac.2017.04.062
Kamal T, Ahmad I, Khan SB, Asiri AM (2018) Agar hydrogel supported metal nanoparticles catalyst for pollutants degradation in water. Desalination Water Treat 136:290–298. https://doi.org/10.5004/dwt.2018.23230
Ali F, Khan SB, Kamal T, Alamry KA, Bakhsh EM, Asiri AM, Sobahi TRA (2018) Synthesis and characterization of metal nanoparticles templated chitosan-SiO2 catalyst for the reduction of nitrophenols and dyes. Carbohydr Polym 192:217–230. https://doi.org/10.1016/j.carbpol.2018.03.029
Ali F, Khan SB, Kamal T, Alamry KA, Asiri AM, Sobahi TRA (2017) Chitosan coated cotton cloth supported zero-valent nanoparticles: simple but economically viable, efficient and easily retrievable catalysts. Sci Rep 7:16957. https://doi.org/10.1038/s41598-017-16815-2
Khan MSJ, Kamal T, Ali F, Asiri AM, Khan SB (2019) Chitosan-coated polyurethane sponge supported metal nanoparticles for catalytic reduction of organic pollutants. Int J Biol Macromol 132:772–783. https://doi.org/10.1016/j.ijbiomac.2019.03.205
Ali N, Azeem S, Khan A, Khan H, Kamal T, Asiri AM (2020) Experimental studies on removal of arsenites from industrial effluents using tridodecylamine supported liquid membrane. Environ Sci Pollut Res 27:11932–11943. https://doi.org/10.1007/s11356-020-07619-5
Ali N, Ismail M, Khan A, Khan H, Haider S, Kamal T (2018) Spectrophotometric methods for the determination of urea in real samples using silver nanoparticles by standard addition and 2nd order derivative methods. Spectrochim Acta Part 189:110–115. https://doi.org/10.1016/j.saa.2017.07.063
Hamidi M, Azadi A, Rafiei P (2008) Hydrogel nanoparticles in drug delivery. Adv Drug Deliv Rev 60:1638–1649. https://doi.org/10.1016/j.addr.2008.08.002
Thomas M, Naikoo GA, Sheikh MUD, Bano M, Khan F (2016) Effective photocatalytic degradation of Congo red dye using alginate/carboxymethyl cellulose/TiO2 nanocomposite hydrogel under direct sunlight irradiation. J Photochem Photobiol Chem 327:33–43. https://doi.org/10.1016/j.jphotochem.2016.05.005
Ghosh SK, Das A, Basu A, Halder A, Das S, Basu S, Abdullah MdF, Mukherjee A, Kundu S (2018) Semi-interpenetrating hydrogels from carboxymethyl guar gum and gelatin for ciprofloxacin sustained release. Int J Biol Macromol 120:1823–1833. https://doi.org/10.1016/j.ijbiomac.2018.09.212
Rocha-García D, Betancourt-Mendiola M de L, Wong-Arce A, Rosales-Mendoza S, Reyes-Hernández J, González-Ortega O, Palestino G (2018) Gelatin-based porous silicon hydrogel composites for the controlled release of tramadol. Eur Polym J 108:485–497. https://doi.org/10.1016/j.eurpolymj.2018.09.033
He Y, Huang G, Pan Z, Liu Y, Gong Q, Yao C, Gao J (2015) Polyelectrolyte induced formation of silver nanoparticles in copolymer hydrogel and their application as catalyst. Mater Res Bull 70:263–271. https://doi.org/10.1016/j.materresbull.2015.04.028
Li L, Wang R, Xing X, Qu W, Chen S, Zhang Y (2019) Preparation of porous semi-IPN temperature-sensitive hydrogel-supported nZVI and its application in the reduction of nitrophenol. J Environ Sci 82:93–102. https://doi.org/10.1016/j.jes.2019.02.024
Yang J, Zhang X, Yu W, Liu W, Bian F (2013) Chelidamic acid functionalized stimuli-responsive hydrogel supported-palladium catalyst for copper-free Sonogashira reaction in aqueous media. React Funct Polym 73:710–718. https://doi.org/10.1016/j.reactfunctpolym.2013.02.007
Shabbir S, Lee Y, Rhee H (2015) Au(III) catalyst supported on a thermoresponsive hydrogel and its application to the A-3 coupling reaction in water. J Catal 322:104–108. https://doi.org/10.1016/j.jcat.2014.11.009
Sahiner N, Ozay O, Aktas N, Inger E, He J (2011) The on demand generation of hydrogen from Co-Ni bimetallic nano catalyst prepared by dual use of hydrogel: as template and as reactor. Int J Hydrog Energy 36:15250–15258. https://doi.org/10.1016/j.ijhydene.2011.08.082
Azeredo HMC, Waldron KW (2016) Crosslinking in polysaccharide and protein films and coatings for food contact—a review. Trends Food Sci Technol 52:109–122. https://doi.org/10.1016/j.tifs.2016.04.008
Hermanto S, Sumarlin LO, Fatimah W (2013) Differentiation of bovine and porcine gelatin based on spectroscopic and electrophoretic analysis. J Food Pharm Sci 1. https://doi.org/10.14499/jfps
Yadollahi M, Farhoudian S, Namazi H (2015) One-pot synthesis of antibacterial chitosan/silver bio-nanocomposite hydrogel beads as drug delivery systems. Int J Biol Macromol 79:37–43. https://doi.org/10.1016/j.ijbiomac.2015.04.032
Elango M, Deepa M, Subramanian R, Musthafa AM (2018) Synthesis, characterization, and antibacterial activity of polyindole/Ag–CuO nanocomposites by reflux condensation method. Polym 57:1440–1451. https://doi.org/10.1080/03602559.2017.1410832
Moosavifard SE, Shamsi J, Fani S, Kadkhodazade S (2014) Facile synthesis of hierarchical CuO nanorod arrays on carbon nanofibers for high-performance supercapacitors. Ceram Int 40:15973–15979. https://doi.org/10.1016/j.ceramint.2014.07.126
Tamuly C, Saikia I, Hazarika M, Das MR (2014) Bio-derived CuO nanocatalyst for oxidation of aldehyde: a greener approach. RSC Adv 4:20636–20640. https://doi.org/10.1039/C4RA01683A
Ali F, Khan SB, Kamal T, Alamry KA, Asiri AM (2018) Chitosan-titanium oxide fibers supported zero-valent nanoparticles: highly efficient and easily retrievable catalyst for the removal of organic pollutants. Sci Rep 8:6260. https://doi.org/10.1038/s41598-018-24311-4
Rehman Sur, Siddiq M, Al-Lohedan H, Sahiner N (2015) Cationic microgels embedding metal nanoparticles in the reduction of dyes and nitro-phenols. Chem Eng J 265:201–209. https://doi.org/10.1016/j.cej.2014.12.061
Khan SB, Khan SA, Marwani HM, Bakhsh EM, Anwar Y, Kamal T, Asiri AM, Akhtar K (2016) Anti-bacterial PES-cellulose composite spheres: dual character toward extraction and catalytic reduction of nitrophenol. RSC Adv 6:110077–110090. https://doi.org/10.1039/c6ra21626a
Santhosh AS, Sandeep S, Kumara Swamy N (2019) Green synthesis of nano silver from euphorbia geniculata leaf extract: Investigations on catalytic degradation of methyl orange dye and optical sensing of Hg2+. Surf Interfaces 14:50–54. https://doi.org/10.1016/j.surfin.2018.11.004
Han D, Li B, Xing G, Zhang Y, Chen Y, Sun Y, Zhang Y, Liu Y, Yang J (2018) Facile synthesis of Fe3Pt-Ag nanocomposites for catalytic reduction of methyl orange. Chem Res Chin Univ 34:871–876. https://doi.org/10.1007/s40242-018-8241-8
Zhang Y, Bai L, Zhou W, Lu R, Gao H, Zhang S (2016) Superior adsorption capacity of Fe3O4@nSiO2@mSiO2 core-shell microspheres for removal of congo red from aqueous solution. J Mol Liq 219:88–94. https://doi.org/10.1016/j.molliq.2016.02.096
Oros-Ruiz S, Gómez R, López R, Hernández-Gordillo A, Pedraza-Avella JA, Moctezuma E, Pérez E (2012) Photocatalytic reduction of methyl orange on Au/TiO2 semiconductors. Catal Commun 21:72–76. https://doi.org/10.1016/j.catcom.2012.01.028
Zhu H-Y, Fu Y-Q, Jiang R, Jiang J-H, Xiao L, Zeng G-M, Zhao S-L, Wang Y (2011) Adsorption removal of congo red onto magnetic cellulose/Fe3O4/activated carbon composite: equilibrium, kinetic and thermodynamic studies. Chem Eng J 173:494–502. https://doi.org/10.1016/j.cej.2011.08.020
Aghakhaninejad S, Rahimi R, Zargari S (2018) Application of BiVO4 nanocomposite for photodegradation of methyl orange. Proc 22nd Int Electron Conf Synth Org Chem 1. https://doi.org/10.3390/ecsoc-22-05666
Ghorai S, Sarkar AK, Panda AB, Pal S (2013) Effective removal of Congo red dye from aqueous solution using modified xanthan gum/silica hybrid nanocomposite as adsorbent. Bioresour Technol 144:485–491. https://doi.org/10.1016/j.biortech.2013.06.108
El-Gamal SMA, Amin MS, Ahmed MA (2015) Removal of methyl orange and bromophenol blue dyes from aqueous solution using Sorel’s cement nanoparticles. J Environ Chem Eng 3:1702–1712. https://doi.org/10.1016/j.jece.2015.06.022
Omidvar A, Jaleh B, Nasrollahzadeh M, Dasmeh HR (2017) Fabrication, characterization and application of GO/Fe3O4/Pd nanocomposite as a magnetically separable and reusable catalyst for the reduction of organic dyes. Chem Eng Res Des 121:339–347. https://doi.org/10.1016/j.cherd.2017.03.026
Wang H, Hu Y, Jiang Y, Qiu L, Wu H, Guo B, Shen Y, Wang Y, Zhu L, Xie A (2013) Facile synthesis and excellent recyclable photocatalytic activity of pine cone-like Fe3O4@Cu2O/Cu porous nanocomposites. Dalton Trans 42:4915. https://doi.org/10.1039/c2dt32290k
Ali N, Awais, Kamal T, Ul-Islam M, Khan A, Shah SJ, Zada A (2018) Chitosan-coated cotton cloth supported copper nanoparticles for toxic dye reduction. Int J Biol Macromol 111:832–838. https://doi.org/10.1016/j.ijbiomac.2018.01.092
Dai J, Fan XM, Liu H, Wang J, Liu HR, Zhang FZ (2012) Fabrication and high visible light photocatalytic properties of Cu/Cu2O nanocomposites by the one-pot solution-phase hydrothermal method. J Nanosci Nanotechnol 12:6412–6419. https://doi.org/10.1166/jnn.2012.6434
Sahoo A, Kumar Tripathy S, Dehury N, Patra S (2015) A porous trimetallic Au@Pd@Ru nanoparticle system: synthesis, characterisation and efficient dye degradation and removal. J Mater Chem A 3:19376–19383. https://doi.org/10.1039/C5TA03959B
Acknowledgements
This work was supported by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah under grant No. (D-200-130-1439). The authors, therefore, gratefully acknowledge the DSR technical and financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmad, S., Khan, S.B., Asiri, A.M. et al. Polypeptide and copper oxide nanocomposite hydrogel for toxicity elimination of wastewater. J Sol-Gel Sci Technol 96, 382–394 (2020). https://doi.org/10.1007/s10971-020-05357-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-020-05357-1