Abstract
To investigate associations of the duration of voriconazole treatment and radiological response with relapse of invasive pulmonary aspergillosis (IPA) in immunocompromised patients, we explored the risk factors for IPA relapse after successful initial treatment. All patients with proven or probable IPA who had finished voriconazole treatment between 2005 and 2019 in a tertiary-care hospital were reviewed. IPA relapse was defined as re-diagnosis of proven or probable IPA at the same site within 1 year after treatment termination. Short course of voriconazole treatment was defined as a treatment less than 9 weeks, which is a median of the recommended minimum duration of therapy from the Infectious Disease Society of America. The radiological response was defined as a reduction in IPA burden by more than 50% on chest computed tomography. Of 87 patients who had completed voriconazole treatment, 14 (16.1%) experienced IPA relapse. Multivariable Cox regression identified that short voriconazole treatment duration (adjusted hazard ratio [aHR], 3.7; 95% confidence interval [CI], 1.1–12.3; P = 0.033) and radiological non-response (aHR, 4.6; 95% CI, 1.2–17.5; P = 0.026) were independently associated with relapse of IPA after adjusting for several clinical risk factors. Longer duration of therapy should be considered for those at higher risk of relapse.
Similar content being viewed by others
Introduction
Invasive pulmonary aspergillosis (IPA) is an infectious disease with a mortality rate of about 30%1,2. Immunocompromised patients with prolonged neutropenia or taking corticosteroids, and those who received allogeneic hematopoietic stem cell transplantation (HCT) or solid-organ transplantation, and with severe influenza are at high risk of IPA3,4. Restoring immune function is crucial for treatment of IPA3,5.
Voriconazole is the drug of choice for IPA1. The recommended treatment duration is a minimum of 6–12 weeks, although there is little evidence for the optimal treatment duration3. In clinical practice, treatment is usually terminated by a physician’s subjective judgement on the patient’s immune status and treatment response. Therapeutic monitoring of IPA includes serial clinical evaluation of symptoms and signs, serial measurement of galactomannan assay (GM) titres and computed tomography (CT) scans3.
A fixed treatment duration for all patients is not possible, since treatment duration needs to be personalized and determined on a patient-basis. Nevertheless, a concern would be the minimum required duration of the voriconazole therapy3,6,7. Insufficient treatment may result in relapse of IPA, which would lead to the increased morbidity and mortality.
In this study, we hypothesized that short course of voriconazole treatment, defined empirically as less than 9 weeks (i.e., median of the commonly recommended duration by the Infectious Disease Society of America3), is associated with relapse of IPA. Therefore, in this retrospective cohort study from a single tertiary medical centre, we analyzed the association between the short treatment duration and relapse of IPA in immunocompromised patients after adjusting for the important clinical confounders using a regression analysis. In addition, we also investigated whether the radiological response at the time of treatment termination was associated with relapse of the disease.
Results
Clinical characteristics of patients with IPA
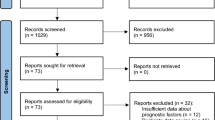
Of 203 patients with proven or probable IPA, 121 had completed voriconazole treatment (Fig. 1). Among these, 34 died (n = 18) or were lost to follow-up (n = 16) within 1 year after treatment. Among all eligible patients (n = 87), 14 (16.1%) experienced IPA relapse. Median duration (interquartile range [IQR]) of voriconazole treatment was 18.9 (12.0–22.4) weeks. Voriconazole accounted for about 90% of the total anti-mold treatment period (median [IQR], 89.5 [86.1–100] in relapse group vs. 89.9 [84.2–100] in non-relapse group, P = 0.516). Median duration (IQR) from the end of treatment to the diagnosis of relapse was 10.8 (4.1–15.3) weeks.
Comparison between patients with and without relapse
The clinical characteristics of the patients according to the relapse of IPA are described in Table 1. Diabetes mellitus was more common in the relapse group than in the non-relapse group (relapse vs. non-relapse; mean, 64.3% vs. 34.2%; P = 0.035), as was a higher Charlson’s comorbidity-weighted index score (mean [± standard deviation], 6.4 [± 1.4] vs. 4.8 [± 2.3]; P = 0.017). Duration of voriconazole treatment tended to be shorter in the relapse group (median week [IQR], 15.8 [8.2–21.5] vs. 19.5 [12.0–22.9]; P = 0.251). Two patients (5.7 weeks in relapse group and 4.7 weeks in non-relapse group, respectively) were treated for less than 6 weeks. Four (28.6%) and 7 (9.6%) patients underwent short course of voriconazole treatment in relapse and non-relapse groups, respectively (P = 0.063).
With respect to the radiological findings, the most common pattern of lung lesions was ground-glass opacity with a halo (78.6% vs. 80.6%; P = 1.000), followed by a nodule or mass (78.6% vs. 79.2%; P = 1.000) regardless of the relapse. The initial number of involved lobes was not significantly different between the two groups (median [IQR], 3 [2–5] vs. 3 [2–5]; P = 0.975). Radiological response (28.6% vs. 61.8%; P = 0.023) was significantly higher in the non-relapse group than in the relapse group, but there was no difference in the rate of complete response (CR) (21.4% vs. 14.7%; P = 0.687). Figure 2 shows representative CT findings from patients with (Fig. 2A,B) or without (Fig. 2C,D) a radiological response.
(A)–(D) Representative computed tomography findings from two patients. The first patient had a consolidation in the right upper lobe (A). A radiological response was achieved after initial treatment (B). The second patient had a nodule in the right lower lobe (C). A radiological response was not achieved (D). Invasive pulmonary aspergillosis relapsed in the second patient.
Independent risk factors for IPA relapse
Multivariable Cox regression analysis identified that short voriconazole treatment duration (adjusted hazard ratio [aHR], 3.7; 95% confidence interval [CI], 1.1–12.3; P = 0.033; Table 2, Fig. 3A,B) and radiological non-response (aHR, 4.6; 95% CI, 1.2–17.5; P = 0.026; Fig. 3C,D) were associated with the relapse of IPA. Detailed results including aHR for the covariates are described in Table 2.
(A)–(D) Kaplan–Meier survival curves showing probability of being IPA-free based on duration of voriconazole therapy (A), and adjusted survival curves (B) after adjusting for data input into multivariable regression. Kaplan–Meier survival curves based on achievement of a radiological response after the end of the IPA treatment (C), and adjusted survival curves (D). IPA invasive pulmonary aspergillosis.
Sensitivity analyses
In the first sensitivity analysis, lack of radiological CR was not associated with relapse of IPA (aHR, 1.1; 95% CI: 0.2–5.8; P = 0.881). However, the aHR (3.9; 95% CI: 1.1–13.5; P = 0.031; Supplementary Table 1) for the short voriconazole treatment duration was significant. The second sensitivity analysis (n = 87), which excluded radiological response in the multivariable analysis, also identified that short voriconazole treatment was an independent risk factor (aHR, 3.9; 95% CI: 1.2–13.3; P = 0.028; Supplementary Table 2). The third sensitivity analysis (n = 72), which included duration of GM positivity instead of a radiological response in the regression, again revealed that the short treatment duration was a significant risk factor (aHR, 4.8; 95% CI: 1.1–21.3; P = 0.041; Supplementary Table 3).
Discussion
In this study, we revealed that voriconazole treatment duration less than 9 weeks and radiological non-response at the time of treatment termination were associated with relapse of IPA. Our results imply that the voriconazole therapy should be continued for at least 9 weeks regardless of the underlying comorbidities in immunocompromised patients. Longer treatment may be indicated based on the patients’ symptoms, underlying medical conditions, and radiological improvement.
Defining appropriate treatment duration of IPA is difficult since it should be decided according to the immune status of the patients and treatment response6. Some guidelines suggest 6–12 weeks as the minimum duration of treatment although supporting evidence is weak3. A clinician should be prudent not to interpret these guidelines that the minimum of 6-week of treatment is sufficient since treatment duration less than 9-week could be risky according to the results of the present study.
Four patients with short voriconazole duration experienced relapse, and the intervals from the end of voriconazole treatment to the diagnosis of relapse in these patients were 41, 68, 92, 101 days, respectively (Fig. 3A). Although it was not possible to molecularly distinguish between relapse and reinfection in the present study8,9, such a short interval between the end of the treatment and re-diagnosis supports that it was a relapse rather than reinfection.
The reliable radiological predictors of treatment of IPA remain unknown. According to Vehrechild et al., an increase of IPA burden in serial chest CT in early course of treatment was a sensitive marker of poor prognosis10. In this study, we investigated the radiological responses using definitions from previous study1, and revealed that the failure to achieve more than 50% reduction of IPA burden was significantly associated with relapse. If the radiological response is not achieved at the time when a clinician had planned to terminate the treatment, an additional course of voriconazole should be considered.
Sensitivity analyses consistently demonstrated that the short voriconazole treatment duration less than 9 weeks was associated with relapse of IPA. Severe underlying illness, a well-known risk factor associated with poor prognosis11,12, was also associated with relapse. Interestingly, radiological CR was not an independent risk factor. However, it is understandable given the fact that only 13 (15.9%) patients achieved radiological CR in our cohort. In this regard, we assume that the radiological response, which includes a broader range of radiological improvement than radiological CR, would be a more appropriate marker to assess the treatment response.
For accurate assessment of aHRs for the variables of interest, we included severity of underlying diseases, immunosuppressive events during IPA treatment, and initial burden of IPA as covariates in the multivariable analysis. These covariates are known as poor prognostic factors of IPA. A retrospective study of 385 patients with invasive aspergillosis and various underlying conditions showed that receipt of allogeneic HCT or solid-organ transplantation, renal impairment, neutropenia, and diffuse pulmonary lesions were predictors of increased overall mortality11. Another study of 405 cases of invasive aspergillosis after HCT identified low pulmonary function, neutropenia, and elevated bilirubin or creatinine levels as independent risk factors for mortality12. Finally, a retrospective study of 27 invasive aspergillosis cases after bone marrow transplantation identified acute or extensive graft-versus-host disease (GVHD) as a risk factor for mortality13.
This study has several limitations. First, it was a retrospective study conducted at a single tertiary-care hospital with a limited number of patients with IPA. Therefore, the results should be validated using a larger prospective cohort. Second, eligible patients had often received long-term voriconazole treatment. Although this may be partly due to unresolved underlying disease, it may have lowered the relapse rate of IPA in our cohort. Third, since the prognosis of IPA depends on the severity of immunosuppression3,5, levels of immunosuppression after initial treatment may be associated with relapse. However, we did not include immunosuppressive events after treatment in the regression analysis as i) the immunosuppressive events during and after treatment are highly correlated with each other, and ii) the variable is not available for a clinician at the time of the treatment termination. Finally, we could not derive an optimal treatment duration for IPA from our study results. Further prospective studies with a larger cohort are warranted to define the optimal duration of therapy or to develop and validate a prediction model to personalize the treatment duration.
In conclusion, short duration of voriconazole therapy and post-treatment radiological non-response were independent risk factors for relapse of IPA. Therefore, voriconazole treatment should be continued for at least 9 weeks in immunocompromised patients and the radiological response should be verified closely at the time of treatment termination.
Methods
Study design
All adults (age \(\ge\) 18 years) with proven or probable IPA14 who had finished voriconazole treatment between January 2005 and July 2019 at Seoul National University Hospital were retrospectively reviewed. Patients who died or were lost to follow-up during initial treatment or within 1 year after the end of the treatment were excluded. Case and control patients comprised those that suffered IPA relapse and those that did not, respectively. The study was approved by the Seoul National University Hospital Institutional Review Board (No. 1909-011-1061). The requirement for written informed consent was waived due to the retrospective nature of the study. All methods were carried out in accordance with the approved guidelines.
Clinical characteristics
The following clinical data were collected from electronic medical records: age, sex and underlying disease (e.g., haematologic diseases [acute myelogenous leukaemia, myelodysplastic syndrome and history of HCT], solid-organ transplantation, or connective tissue disease, and history of diabetes mellitus or chronic lung disease). The severity of comorbidities was measured using the Charlson’s comorbidity-weighted index score15,16. It was also reviewed if the patient had experienced severe neutropenia, GVHD, or rejection during treatment for IPA. The duration of voriconazole or other anti-mold treatments was also noted.
Serum GM was measured using an enzyme-linked immunosorbent assay (Platelia Aspergillus kit) according to the manufacturer’s instructions (Bio-Rad Laboratories, Hercules, CA, USA). The threshold optical density index of Aspergillus galactomannan was set at 0.517. Aspergillus antigen-positive week, the period until GM titres became less than 0.5, was reviewed.
Chest CT scans were reviewed at the start and end of antifungal treatment, and the characteristics (i.e., ground-glass opacity, consolidation or mass with or without cavitation) and burden of IPA lesions were noted. The sums of the longest diameters of up to three lung nodules, the number of nodules and the number of involved lobes were recorded as surrogates for IPA burden. Also, changes in IPA burden before and after the treatment were reviewed.
Definitions
A definition of proven or probable IPA was made in accordance with the revised criteria of the European Organization for Research and Treatment of Cancer/Mycoses Study Group14. Relapse was defined as a re-diagnosis of proven or probable IPA at the same site with or without dissemination within 1 year after the end of initial voriconazole treatment, according to Cornely et al.18. If initial lesions were not completely resolved at treatment termination, aggravation with additional evidence of proven or probable IPA (positive GM or culture with appropriate clinical context) was regarded as a relapse. Time from the end of treatment to diagnosis of relapse was calculated.
Neutropenia was defined as an absolute neutrophil count of < 500/μL for more than 1 week19,20. Definitions of GVHD21,22 and rejection23,24,25 in patients who underwent HCT or solid-organ transplantation were the same as in previous reports. Radiological response was defined as more than 50% reduction in the IPA burden without aggravation of original lesions or occurrence of new lesions1. Radiological CR was defined as disappearance of all lung lesions. Duration of voriconazole treatment was dichotomized arbitrarily into two groups, with 9 weeks as the threshold (i.e., median value of commonly recommended duration by the Infectious Disease Society of America3).
Statistical analysis
The clinical and microbiological characteristics of patients with and without IPA relapse were compared. According to results of the Shapiro–Wilk test, the Student’s t-test or Mann–Whitney U-test was used to compare continuous variables. The Chi-squared test or Fisher’s exact test was used to compare categorical variables.
Multivariable Cox regression analysis was conducted to identify independent risk factors for relapse after treatment26. Duration from the end of initial treatment to diagnosis of relapse was used as a time variable. Inclusion of variables in multivariable analyses was based on a priori clinical knowledge of the risk factors for poor prognosis in patients with IPA27,28. Specifically, impaired renal or liver function11,12; immunosuppressive events such as neutropenia, GVHD or rejection11,13,29,30; and the number of lung lobes involved initially11 were analyzed as covariates, in addition to radiological treatment responses10 and duration of voriconazole treatment. Multicollinearity was checked using variance inflation factors, with 5 set as the cut-off. Unadjusted Kaplan–Meier survival curves were drawn after identification of the independent risk factors. In addition, a marginal approach with reweighted data was used to construct adjusted survival curves31.
Sensitivity analyses were performed in three ways. First, multivariable analysis was performed using CR rather than the whole radiological response as a treatment response variable. Second, to eliminate confounding effects introduced by patients lacking follow-up CT scans, analysis was conducted after excluding the radiological response. Lastly, Aspergillus antigen-positive week was added instead of a radiological response in the regression analysis as a surrogate for the therapeutic effect.
All analyses were performed using PASW for Windows (version 25.0; SPSS Inc., Chicago, IL, USA). Survival curves were plotted using R software version 3.5.1 (https://www.R-project.org; survminer package). A P value < 0.05 was considered statistically significant.
Data availability
The data supporting this publication can be accessed by contacting the corresponding authors on reasonable request.
References
Herbrecht, R. et al. Voriconazole versus amphotericin b for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347, 408–415 (2002).
Maertens, J. et al. Treatment outcomes in patients with proven/probable vs possible invasive mould disease in a phase III trial comparing isavuconazole vs voriconazole. Mycoses 61, 868–876 (2018).
Patterson, T. F. et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases Society of America. Clin. Infect. Dis. 63, e1–e60 (2016).
Schauwvlieghe, A. F. A. D. et al. Invasive aspergillosis (Bala et al. 2018)patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir. Med. 6, 782–792 (2018).
Thompson, G. R. & Patterson, T. F. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases 9th edn, 3109–3116 (Elsevier, Amsterdam, 2020).
Ullmann, A. J. et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect, 24(Suppl 1), e1–e38 (2018).
Herbrecht, R. et al. Treatment of invasive Candida and invasive Aspergillus infections in adult haematological patients. Eur. J. Cancer 5, 49–59 (2007).
Sipsas, N. V. & Kontoyiannis, D. P. Clinical issues regarding relapsing aspergillosis and the efficacy of secondary antifungal prophylaxis in patients with hematological malignancies. Clin. Infect. Dis. 42, 1584–1591 (2006).
Cadranel, J. et al. Voriconazole for chronic pulmonary aspergillosis: a prospective multicenter trial. Eur. J. Clin. Microbiol. Infect. Dis. 31, 3231–3239 (2012).
Vehreschild, J. J. et al. Serial assessment of pulmonary lesion volume by computed tomography allows survival prediction in invasive pulmonary aspergillosis. Eur. Radiol. 27, 3275–3282 (2017).
Nivoix, Y. et al. Factors associated with overall and attributable mortality in invasive aspergillosis. Clin. Infect. Dis. 47, 1176–1184 (2008).
Upton, A., Kirby, K. A., Carpenter, P., Boeckh, M. & Marr, K. A. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin. Infect. Dis. 44, 531–540 (2007).
Ribaud, P. et al. Survival and prognostic factors of invasive aspergillosis after allogeneic bone marrow transplantation. Clin. Infect. Dis. 28, 322–330 (1999).
De Pauw, B. et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46, 1813–1821 (2008).
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 40, 373–383 (1987).
Schneeweiss, S., Wang, P. S., Avorn, J. & Glynn, R. J. Improved comorbidity adjustment for predicting mortality in Medicare populations. Health Serv. Res. 38, 1103–1120 (2003).
Wheat, L. J. & Walsh, T. J. Diagnosis of invasive aspergillosis by galactomannan antigenemia detection using an enzyme immunoassay. Eur. J. Clin. Microbiol. Infect. Dis. 27, 245–251 (2008).
Cornely, O. A. et al. Defining breakthrough invasive fungal infection—position paper of the mycoses study group education and research consortium and the European Confederation of Medical Mycology. Mycoses 62, 716–729 (2019).
de Naurois, J. et al. Management of febrile neutropenia: ESMO Clinical Practice Guidelines. Ann. Oncol. 21, v252–v256 (2010).
Kuderer, N. M., Dale, D. C., Crawford, J., Cosler, L. E. & Lyman, G. H. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106, 2258–2266 (2006).
Rowlings, P. A. et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br. J. Haematol. 97, 855–864 (1997).
Akpek, G. et al. Performance of a new clinical grading system for chronic graft-versus-host disease: a multicenter study. Blood 102, 802–809 (2003).
Stewart, S. et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J. Heart Lung Transpl. 26, 1229–1242 (2007).
Demetris, A. J. et al. Banff schema for grading liver allograft rejection: an international consensus document. Hepatology 25, 658–663 (1997).
Solez, K. I. M. et al. International standardization of criteria for the histologic diagnosis of renal allograft rejection: the Banff working classification of kidney transplant pathology. Kidney Int. 44, 411–422 (1993).
Lin, D. Cox regression analysis of multivariate failure time data: the marginal approach. Stat. Med. 13, 2233–2247 (1994).
Sun, G. W., Shook, T. L. & Kay, G. L. Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. J. Clin. Epidemiol. 49, 907–916 (1996).
Moons, K. G. M. et al. PROBAST: a tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Ann. Intern. 170, W1–W33 (2019).
Kim, S.-H. et al. Epidemiology and clinical outcomes of invasive pulmonary aspergillosis: a nationwide multicenter study in Korea. Infect. Chemother. 44, 282–288 (2012).
Cordonnier, C. et al. Prognostic factors for death due to invasive aspergillosis after hematopoietic stem cell transplantation: a 1-year retrospective study of consecutive patients at French transplantation centers. Clin. Infect. Dis. 42, 955–963 (2006).
Seaman, S. R. & White, I. R. Review of inverse probability weighting for dealing with missing data. Stat. Methods Med. Res. 22, 278–295 (2011).
Acknowledgements
Activities not related to the present article: HK received a research grant from Lunit Inc. (Seoul, South Korea).
Author information
Authors and Affiliations
Contributions
H.K. and C.K.K. conceived and designed the study, and have full access to all of the data; D.H.S., S.-J.Y., K.I.J., H.K., K.-H.S., P.G.C., W.B.P., J.-H.B., E.S.K., S.W.P., H.B.K., N.-J.K. and M.-dO. collected clinical data; D.H.S., S.-J.Y., H.K. and C.K.K. analyzed the data; D.H.S. and S.-J.Y. wrote the first draft of the manuscript; all authors participated in subsequent revisions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shin, D.H., Yoo, SJ., Jun, K.I. et al. Short course of voriconazole therapy as a risk factor for relapse of invasive pulmonary aspergillosis. Sci Rep 10, 16078 (2020). https://doi.org/10.1038/s41598-020-73098-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-73098-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.