Abstract
Purpose
A combined in vitro – in silico methodology was designed to estimate pharmacokinetics of budesonide delivered via dry powder inhaler.
Methods
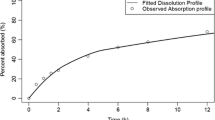
Particle size distributions from three budesonide DPIs, measured with a Next Generation Impactor and Alberta Idealized Throat, were input into a lung deposition model to predict regional deposition. Subsequent systemic exposure was estimated using a pharmacokinetic model that incorporated Nernst-Brunner dissolution in the conducting airways to predict the net influence of dissolution, mucociliary clearance, and absorption.
Results
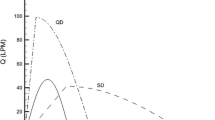
DPIs demonstrated significant in vitro differences in deposition, resulting in large differences in simulated regional deposition in the central conducting airways and the alveolar region. Similar but low deposition in the small conducting airways was observed with each DPI. Pharmacokinetic predictions showed good agreement with in vivo data from the literature. Peak systemic concentration was tied primarily to the alveolar dose, while the area under the curve was more dependent on the total lung dose. Tracheobronchial deposition was poorly correlated with pharmacokinetic data.
Conclusions
Combination of realistic in vitro experiments, lung deposition modeling, and pharmacokinetic modeling was shown to provide reasonable estimation of in vivo systemic exposure from DPIs. Such combined approaches are useful in the development of orally inhaled drug products.






Similar content being viewed by others
Abbreviations
- DPI:
-
Dry powder inhaler
- MMAD:
-
Mass median aerodynamic diameter
- NGI:
-
Next Generation Impactor
- AUC 24 :
-
Area under the curve (24 h)
- F BA :
-
Oral bioavailability
- F i :
-
Fraction of dose depositing in ith compartment
- K diss,TB :
-
Effective dissolution rate in tracheobronchial region
- P m :
-
Measured Pressure
- P ref :
-
Reference Pressure
- Q peak :
-
Peak inhalation flowrate
- T m :
-
Measured Temperature
- T ref :
-
Reference Temperature
- V ASL,i :
-
Volume of airway surface liquid in ith airway compartment
- V C :
-
Volume of central compartment
- V d,ss :
-
Volume of distribution at steady state
- c i :
-
Drug concentration in ith airway compartment
- c max :
-
Maximum serum concentration in central compartment
- c s :
-
Drug solubility
- d g,50 :
-
Particle geometric mean diameter
- k 10 :
-
Elimination rate constant
- k 12 :
-
Central to peripheral rate constant
- k 21 :
-
Peripheral to central rate constant
- k a :
-
Oral absorption rate constant
- k ALV :
-
Alveolar region absorption rate constant
- k diss,ALV :
-
Dissolution rate constant in alveolar region
- k muc,i :
-
Mucociliary rate constant for ith airway compartment
- k TB :
-
Tracheobronchial region absorption rate constant
- m i,1 :
-
Drug mass (solid) in ith airway compartment
- m i,2 :
-
Drug mass (dissolved) in ith airway compartment
- t max :
-
Time at which maximum serum concentration occurs
- t total :
-
Duration of inhalation
- ρ P :
-
Particle density
- h :
-
Diffusion layer thickness
- CL :
-
Clearance
- D :
-
Diffusion coefficient
- Q :
-
Flowrate
- R :
-
Inhaler resistance
- S :
-
Surface area of particles undergoing dissolution
- c :
-
Drug concentration
- m :
-
Drug mass
- t :
-
Time
- A:
-
Gastrointestinal tract compartment
- ALV:
-
Alveolar
- ASL:
-
Airway surface liquid
- DPI:
-
At the inlet of the inhaler
- DPI exit:
-
Immediately downstream of inhaler mouthpiece
- HBM:
-
Breathing machine line
- P:
-
Peripheral compartment
- std:
-
Standard flowrate
- supply:
-
Building air supply line
- TB:
-
Tracheobronchial
- vacuum:
-
Vacuum line
- vol:
-
Volumetric flowrate
- X:
-
Central compartment
References
Hossny E, Rosario N, Lee BW, Singh M, El-Ghoneimy D, Soh JY, et al. The use of inhaled corticosteroids in pediatric asthma: update. World Allergy Organ J. 2016;9(1):1–24.
Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–73.
Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. Am J Respir Crit Care Med. 2017;195(5):557–82.
Pittas AG, Westcott GP, Balk EM. Efficacy, safety, and patient acceptability of Technosphere inhaled insulin for people with diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3(11):886–94.
San L, Estrada G, Oudovenko N, Montañés F, Dobrovolskaya N, Bukhanovskaya O, et al. PLACID study: a randomized trial comparing the efficacy and safety of inhaled loxapine versus intramuscular aripiprazole in acutely agitated patients with schizophrenia or bipolar disorder. Eur Neuropsychopharmacol. 2018;28(6):710–8.
Olanow CW, Stocchi F. Levodopa: a new look at an old friend. Mov Disord. 2018;33(6):859–66.
Martin AR, Moore CP, Finlay WH. Models of deposition, pharmacokinetics, and intersubject variability in respiratory drug delivery. Expert Opin Drug Deliv. 2018;15(12):1175–88.
Finlay WH. The mechanics of inhaled pharmaceutical aerosols : an introduction. 2nd ed. San Diego: Academic Press; 2019. 306 p.
Ruge CC, Kirch J, Lehr CM. Pulmonary drug delivery: from generating aerosols to overcoming biological barriers-therapeutic possibilities and technological challenges. Lancet Respir Med. 2013;1(5):402–13.
Loira-Pastoriza C, Todoroff J, Vanbever R. Delivery strategies for sustained drug release in the lungs. Adv Drug Deliv Rev. 2014;75:81–91.
Koullapis P, Kassinos SC, Muela J, Perez-Segarra C, Rigola J, Lehmkuhl O, et al. Regional aerosol deposition in the human airways: the SimInhale benchmark case and a critical assessment of in silico methods. Eur J Pharm Sci. 2018;113(June 2017):77–94.
Walenga RL, Babiskin AH, Zhao L. In Silico methods for development of generic drug–device combination orally inhaled drug products. CPT Pharmacometrics Syst Pharmacol. 2019;8(6):359–70.
Wei W, Hindle M, Kaviratna A, Huynh B, Delvadia R, Sandell D, et al. In Vitro Tests for Aerosol Deposition . VI : Realistic Testing with Different Mouth – Throat Models and In Vitro — In Vivo Correlations for a Dry Powder. J Aerosol Med Pulm Drug Deliv. 2018;31(0):1–14.
Ruzycki CA, Martin AR, Finlay WH. An exploration of factors affecting in vitro deposition of pharmaceutical aerosols in the Alberta idealized throat. J Aerosol Med Pulm Drug Deliv. 2019;32(6):405–17.
Bhagwat S, Schilling U, Chen MJ, Wei X, Delvadia R, Absar M, et al. Predicting pulmonary pharmacokinetics from in vitro properties of dry powder inhalers. Pharm Res. 2017;34(12):2541–56.
Bäckman P, Tehler U, Olsson B. Predicting exposure after Oral inhalation of the selective glucocorticoid receptor modulator, AZD5423, based on dose, deposition pattern, and mechanistic modeling of pulmonary disposition. J Aerosol Med Pulm Drug Deliv. 2017;30(2):108–17.
Boger E, Fridén M. Physiologically based pharmacokinetic/Pharmacodynamic modeling accurately predicts the better Bronchodilatory effect of inhaled versus Oral salbutamol dosage forms. J Aerosol Med Pulm Drug Deliv. 2019;32(1):1–12.
Caniga M, Cabal A, Mehta K, Ross DS, Gil MA, Woodhouse JD, et al. Preclinical experimental and mathematical approaches for assessing effective doses of inhaled drugs, using Mometasone to support human dose predictions. J Aerosol Med Pulm Drug Deliv. 2016;29(4):362–77.
Martin AR, Finlay WH. Model calculations of regional deposition and disposition for single doses of inhaled liposomal and dry powder ciprofloxacin. J Aerosol Med Pulm Drug Deliv. 2018;31(1):49–60.
Weber B, Hochhaus G. A pharmacokinetic simulation tool for inhaled corticosteroids. Am Assoc Pharm Sci J. 2013;15(1):159–71.
Byron PR. Prediction of drug residence times in regions of the human respiratory tract following aerosol inhalation. J Pharm Sci. 1986;75(5):433–8.
Gonda I. Drugs administered directly into the respiratory tract: modeling of the duration. J Pharm Sci. 1988;77(4):340–6.
Soulele K, Macheras P, Karalis V. On the pharmacokinetics of two inhaled budesonide/formoterol combinations in asthma patients using modeling approaches. Pulm Pharmacol Ther. 2018;48(July 2017):168–78.
Lee SL, Saluja B, García-Arieta A, Santos GML, Li Y, Lu S, et al. Regulatory considerations for approval of generic inhalation drug products in the US, EU, Brazil, China, and India. AAPS J. 2015;17(5):1285–304.
Lu D, Lee SL, Lionberger RA, Choi S, Adams W, Caramenico HN, et al. International guidelines for bioequivalence of locally acting orally inhaled drug products: similarities and differences. AAPS J. 2015;17(3):546–57.
Delvadia RR, Wei X, Longest PW, Venitz J, Byron PR. In vitro tests for aerosol deposition. IV: simulating variations in human breath profiles for realistic DPI testing. J Aerosol Med Pulm Drug Deliv. 2016;29(2):196–206.
Weers J, Clark A. The impact of inspiratory flow rate on drug delivery to the lungs with dry powder inhalers. Pharm Res. 2017;34(3):507–28.
United States Pharmacopeia. USP 44(5) General Chapter <601> Inhalation and Nasal Drug Products - Aerosols, Sprays, and Powders - Performance Quality Tests. 2019.
United States Pharmacopeia. USP 45(2) Informative Chapter <1604> Data Interpretation of Aerodynamic Particle Size Distribution Measurements for Orally Inhaled Products. 2019.
Hinds WC. Aerosol technology : properties, behavior, and measurement of airborne particles. 2nd ed. Hoboken, NJ: Wiley; 1999.
Javaheri E, Shemirani FM, Pichelin M, Katz IM, Caillibotte G, Vehring R, et al. Deposition modeling of hygroscopic saline aerosols in the human respiratory tract : comparison between air and helium – oxygen as carrier gases. J Aerosol Sci. 2013;64:81–93.
Finlay WH, Lange CF, King M, Speert DP. Lung Delivery of Aerosolized Dextran. 2000;161:91–7.
International Commission on Radiological Protection. Human respiratory tract model for radiological protection : a report of a task group of the International Commission on Radiological Protection. Vols. 24 1–3. Oxford, Eng. :Tarrytown, N.Y.: published for the International Commission on Radiological Protection by Pergamon; 1994. 482 p.
Chan TL, Lippmann M. Experimental measurements and empirical modelling of the regional deposition of inhaled particles in humans. Am Ind Hyg Assoc J. 1980;41:399–409.
Lange CF, Hancock REW, Samuel J, Finlay WH. In vitro aerosol delivery and regional airway surface liquid concentration of a liposomal cationic peptide. J Pharm Sci. 2001;90(10):1647–57.
May S, Jensen B, Weiler C, Wolkenhauer M, Schneider M, Lehr CM. Dissolution testing of powders for inhalation: influence of particle deposition and modeling of dissolution profiles. Pharm Res. 2014;31(11):3211–24.
Ryrfeldt A, Andersson P, Edsbacker S, Tonnesson M, Davies D, Pauwels R. Pharmacokinetics and metabolism of budesonide, a selective glucocorticoid. Eur J Respir Dis. 1982;63(S 122):86–95.
Thorsson L, Edsbacker S, Conradson TB. Lung deposition of budesonide from Turbuhaler® is twice that from a pressurized metered-dose inhaler P-MDI. Eur Respir J. 1994;7(10):1839–44.
Hochhaus G, Möllmann H, Derendorf H, Gonzalez-Rothi RJ. Pharmacokinetic/pharmacodynamic aspects of aerosol therapy using glucocorticoids as a model. J Clin Pharmacol. 1997;37(10):881–92.
Yates JWT, Arundel PA. On the volume of distribution at steady state and its relationship with two-compartmental models. J Pharm Sci. 2008;97(1):111–22.
Dokoumetzidis A, Macheras P. A century of dissolution research: from Noyes and Whitney to the biopharmaceutics classification system. Int J Pharm. 2006;321(1–2):1–11.
Schürch S, Gehr P, Im Hof V, Geiser M, Green F. Surfactant displaces particles toward the epithelium in airways and alveoli. Respir Physiol. 1990;80(1):17–32.
Hastedt JE, Bäckman P, Clark AR, Doub W, Hickey A, Hochhaus G, et al. Scope and relevance of a pulmonary biopharmaceutical classification system AAPS/FDA/USP workshop march 16-17th, 2015 in Baltimore. MD Am Assoc Pharm Sci Open. 2016;2(1):1.
Hintz RJ, Johnson KC. The effect of particle size distribution on dissolution rate and oral absorption. Int J Pharm. 1989;51(1):9–17.
Argenti D, Shah B, Heald D. A study comparing the clinical pharmacokinetics, pharmacodynamics, and tolerability of triamcinolone acetonide HFA-134a metered-dose inhaler and budesonide dry-powder inhaler following inhalation administration. J Clin Pharmacol. 2000;40(5):516–26.
Duddu SP, Sisk SA, Walter YH, Tarara TE, Trimble KR, Clark AR, et al. Improved lung delivery from a passive dry powder inhaler using an engineered PulmoSphere® powder. Pharm Res. 2002;19(5):689–95.
Harrison TW, Tattersfield AE. Plasma concentrations of fluticasone propionate and budesonide following inhalation from dry powder inhalers by healthy and asthmatic subjects. Thorax. 2003;58(3):258–60.
Lähelmä S, Kirjavainen M, Kela M, Herttuainen J, Vahteristo M, Silvasti M, et al. Equivalent lung deposition of budesonide in vivo: a comparison of dry powder inhalers using a pharmacokinetic method. Br J Clin Pharmacol. 2005;59(2):167–73.
Mollmann H, Wagner M, Krishnaswami S, Dimova H, Tang Y, Falcoz C, et al. Single-dose and steady-state pharmacokinetic and pharmacodynamic evaluation of therapeutically clinically equivalent doses of inhaled fluticasone propionate and budesonide, given as diskus?? Or turbohaler?? Dry-powder inhalers to healthy subjects. J Clin Pharmacol. 2001;41(12):1329–38.
Thorsson L, Edsbacker S, Kallen A, Lofdahl C-G. Pharmacokinetics and systemic activity of fluticasone via Diskus and pMDI, and of budesonide via Turbuhaler. Br J Clin Pharmacol. 2001;52:529–38.
Mortimer KJ, Tattersfield AE, Tang Y, Wu K, Lewis S, Hochhaus G, et al. Plasma concentrations of fluticasone propionate and budesonide following inhalation: effect of induced bronchoconstriction. Br J Clin Pharmacol. 2007;64(4):439–44.
Hämäläinen KM, Granander M, Toivanen P, Malinen A. Assessment of the systemic effects of budesonide inhaled from Easyhaler® and from Turbuhaler® in healthy male volunteers. Respir Med. 2001;95(11):863–9.
Mylan Products Ltd UK. Budelin Novolizer 200 micrograms per actuation inhalation powder [Internet]. 2018 [cited 2020 Jun 22]. Available from: https://www.medicines.org.uk/emc/product/9715/smpc
Meda Pharma GmbH. Budelin Novolizer 200 mikrogramov/odmerek prašek za inhaliranje [Internet]. 2017 [cited 2020 Jun 22]. Available from: http://www.cbz.si/cbz/bazazdr2.nsf/o/B0174ADF66EBFFE9C12579C2003F4EC8/$File/s-018681.pdf
Kaiser H, Aaronson D, Dockhorn R, Edsbäcker S, Korenblat P, Källén A. Dose-proportional pharmacokinetics of budesonide inhaled via Turbuhaler®. Br J Clin Pharmacol. 1999;48(3):309–16.
Parisini I, Cheng SJ, Symons DD, Murnane D. Potential of a cyclone prototype spacer to improve in vitro dry powder delivery. Pharm Res. 2014;31(5):1133–45.
Yoshida H, Kuwana A, Shibata H, Izutsu K, Goda Y. Comparison of aerodynamic particle size distribution between a next generation Impactor and a Cascade Impactor at a range of flow rates. AAPS PharmSciTech. 2017;18(3):646–53.
Wei X, Hindle M, Delvadia RR, Byron PR. In Vitro Tests for Aerosol Deposition. V: Using Realistic Testing to Estimate Variations in Aerosol Properties at the Trachea. J Aerosol Med Pulm Drug Deliv. 2017;30(5):jamp.2016.1349.
Dehaan WH, Finlay WH. Predicting extrathoracic deposition from dry powder inhalers. J Aerosol Sci. 2004;35:309–31.
Grgic B, Finlay WH, Burnell PKP, Heenan AF. In vitro intersubject and intrasubject deposition measurements in realistic mouth – throat geometries. J Aerosol Sci. 2004;35:1025–40.
Walenga RL, Longest PW. Current inhalers deliver very small doses to the lower tracheobronchial airways: assessment of healthy and constricted lungs. J Pharm Sci. 2016;105(1):147–59.
Hamid Q, Song Y, Kotsimbos TC, Minshall E, Bai TR, Hegele RG, et al. Inflammation of small airways in asthma. J Allergy Clin Immunol. 1997;100(1):44–51.
N. Richard, Dekhuijzen P. Anti-Inflammatory Drug Targeting in Asthma. Should Inhaled Corticosteroids Reach the Small Airways? Curr Drug ther. 2013;7(4):248–54.
Van Den Berge M, Ten Hacken NHT, Van Der Wiel E, Postma DS. Treatment of the bronchial tree from beginning to end: targeting small airway inflammation in asthma. Allergy Eur J Allergy Clin Immunol. 2013;68(1):16–26.
Ostrovski Y, Dorfman S, Mezhericher M, Kassinos S, Sznitman J. Targeted drug delivery to upper airways using a pulsed aerosol bolus and inhaled volume tracking method. Flow, Turbul Combust. 2019;102(1):73–87.
Tian G, Longest PW, Su G, Hindle M. Characterization of respiratory drug delivery with enhanced condensational growth using an individual path model of the entire tracheobronchial airways. Ann Biomed Eng. 2011;39(3):1136–53.
Kopsch T, Murnane D, Symons D. Optimizing the entrainment geometry of a dry powder inhaler: methodology and preliminary results. Pharm Res. 2016;33(11):2668–79.
Borgstrom L, Bondesson E, Moren F, Trofast E, Newman SP. Lung deposition of budesonide inhaled via Turbuhaler®: a comparison with terbutaline sulphate in normal subjects. Eur Respir J. 1994;7(1):69–73.
Newman SP, Pitcairn GR, Hirst PH, Bacon RE, O’Keefe E, Reiners M, et al. Scintigraphic comparison of budesonide deposition from two dry powder inhalers. Eur Respir J. 2000;16(1):178–83.
Hirst PH, Bacon RE, Pitcairn GR, Silvasti M, Newman SP. A comparison of the lung deposition of budesonide from Easyhlaler®, Turbuhaler® and pMDI plus spacer in asthmatic patients. Respir Med. 2001;95(9):720–7.
Hirst PH, Newman SP, Clark DA, Hertog MGL. Lung deposition of budesonide from the novel dry powder inhaler Airmax™. Respir Med. 2002;96(6):389–96.
Fleming J, Conway J, Majoral C, Katz I, Caillibotte G, Pichelin M, et al. Controlled, parametric, individualized, 2-D and 3-D imaging measurements of aerosol deposition in the respiratory tract of asthmatic human subjects for model validation. J Aerosol Med Pulm Drug Deliv. 2015;28(6):432–51.
Smith DJ, Gaffney EA, Blake JR. Modelling mucociliary clearance. Respir Physiol Neurobiol. 2008;163(1–3):178–88.
Eriksson J, Thörn H, Sjögren E, Holmstén L, Rubin K, Lennernäs H. Pulmonary dissolution of poorly soluble compounds studied in an ex vivo rat lung model. Mol Pharm. 2019;16:3053–64.
Bäckman P, Olsson B. Pulmonary Drug Dissolution, Regional Retention and Systemic Absorption: Understanding their Interactions Through Mechanistic Modeling. Respir Drug Deliv 2020. 2020;50:113–22.
Floroiu A, Klein M, Krämer J, Lehr CM. Towards standardized dissolution techniques for in vitro performance testing of dry powder inhalers. Dissolution Technol. 2018;25(3):6–18.
Hochhaus G, Chen M, Shur J, Kurumaddali A, Schilling U, Jiao Y, et al. Unraveling the pulmonary fate of fluticasone and friends : meeting the physiologic and pharmacokinetic challenges. Respir Drug Deliv. 2020;2020:139–46.
Bäckman P, Arora S, Couet W, Forbes B, de Kruijf W, Paudel A. Advances in experimental and mechanistic computational models to understand pulmonary exposure to inhaled drugs. Eur J Pharm Sci. 2018;113(June 2017):41–52.
Olsson B, Borgström L, Lundbäck H, Svensson M. Validation of a general in vitro approach for prediction of Total lung deposition in healthy adults for pharmaceutical inhalation products. J Aerosol Med Pulm Drug Deliv. 2013;26(6):355–69.
Chuchalin AG, Kremer H-J, Metzenauer P, O’Keefe E, Hermann R. Clinical equivalence trial on budesonide delivered either by the Novolizer® multidose dry powder inhaler or the Turbuhaler® in asthmatic patients. Respiration. 2002;69(6):502–8.
Vanto T, Hämäläinen KM, Vahteristo M, Wille S, Njå F, Hyldebrandt N. Comparison of two budesonide dry powder inhalers in the treatment of asthma in children. J Aerosol Med Depos Clear Eff Lung. 2004;17(1):15–24.
Schweisfurth H, Malinen A, Koskela T, Toivanen P, Ranki-Pesonen M. Comparison of two budesonide powder inhalers, Easyhaler® and Turbuhaler®, in steroid-naïve asthmatic patients. Respir Med. 2002;96(8):599–606.
Van Den Brink KIM, Boorsma M, Staal-Van Den Brekel AJ, Edsbäcker S, Wouters EF, Thorsson L. Evidence of the in vivo esterification of budesonide in human airways. Br J Clin Pharmacol. 2008;66(1):27–35.
Shao J, Talton J, Wang Y, Winner L, Hochhaus G. Quantitative assessment of pulmonary targeting of inhaled corticosteroids using ex vivo receptor binding studies. AAPS J. 2020;22(2):1–10.
Ruzycki CA, Yang M, Chan H-K, Finlay WH. Improved prediction of intersubject variability in extrathoracic aerosol deposition using algebraic correlations. Aerosol Sci Technol. 2017;51(6):667–73.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1 – Volumetric Flowrates
The Delvadia et al. semi-idealized inhalation profiles are presented in terms of the volumetric flowrate exiting the inhaler mouthpiece. The setup in Fig. 1 can provide an indirect measure of this flowrate by considering a mass balance of flow. Consider a control volume encompassing the supply line, the breathing machine line, the vacuum line downstream of the NGI, and the airflow entering the DPI. The equation for conservation of mass in this control volume is: \( \frac{dm}{dt}=\sum {\dot{m}}_{\mathrm{in}}-\sum {\dot{m}}_{\mathrm{out}} \) (A-1).
The time rate of change of mass inside the control volume, dm/dt, is considered negligible relative to the magnitudes of the inlet and outlet flows. This assumption is justified by noting that all flows here have Mach numbers less than 0.3 (i.e. flow can be considered incompressible, so changes in density are small) and the walls of the control volume are rigid (i.e. the actual volume of gas contained in the control volume remains constant). Noting that m = ρV (mass equals density times volume), expanding with the product rule for differentiation, and using the above physical reasoning (incompressible flow and a rigid control volume), dm/dt is:
The equation for conservation of mass becomes, after expressing the inlet and outlet flows in terms of their volumetric flowrates Qvol,supply, Qvol,HBM, Qvol,vacuum, and Qvol,DPI:
Equation A - 3 can be recast in terms of standard flowrates using the ideal gas law as follows. The volumetric flowrate at a particular temperature and pressure relates to the standard flowrate as
From the ideal gas law:
Equation A - 4 can then be expressed as
Here ρm is the air density at which the volumetric flowrate is desired (dependent on temperature and pressure), while ρref is a reference density (equal to approximately 1.2 kg/m^3 for TSI calibrated flowmeters). With suitable substitutions, Eq. A - 3 takes a simple form as all density terms become ρref. Further rearranging to solve for the unmeasured flowrate entering the DPI, Eq. A - 3 becomes:
Flowrates on the right hand side of Eq. A - 7 are known, allowing for the straightforward calculation of the standard flowrate generated through the DPI, Qstd, DPI. Calculation of the volumetric flowrate exiting the DPI mouthpiece can then be performed using A - 8 (Ruzycki et al., J Aerosol Med Pulm Drug Deliv 2019;32 (6):405–417).
Pref equals 101.3 kPa, Tref equals 21.11°C (294.26 K), and R is the device resistance (taken as the reference value measured at sea level). Eq. A - 8 assumes that the effect of ambient pressure on inhaler resistance is negligible (reasonable for moderate altitudes; Titosky et al., J Pharm Sci 2014;103:2116–2124; Ruzycki et al., J Aerosol Med Pulm Drug Deliv 2018;31 (4):221–236). Furthermore, the derivation assumes that the relation between pressure drop and flowrate is quasi-steady, a reasonable assumption given the small volume of the inhaler relative to the inhalation flowrate.
Appendix 2 – Equations Describing the Pharmacokinetic Model
The equations describing the pharmacokinetic model shown schematically in Fig. 2 of the main text are summarized in this Appendix. Note that initial deposited masses in each generation of the tracheobronchial airways and in the alveolar region (Fi, 0 ≤ i ≤ 14, and FALV, respectively) come directly from the regional deposition model discussed in the main text, while the initial dose in the gastrointestinal tract is taken as the dose measured in the Alberta Idealized Throat in vitro. Rate constants describing mucociliary clearance (kmuc,i) and the volume of the airway surface liquid in each generation VASL,i come from the airway surface liquid model discussed in the main text. Values for other rate constants and critical parameters are provided in the main text with references to the literature.
Gastrointestinal tract compartment drug mass, m A :
Equation B - 1 is subject to the initial condition mA equal to the dose measured in the Alberta Idealized Throat at time t = 0
Central compartment drug mass, m X :
Equation B - 2 is subject to the initial condition mX = 0 at time t = 0
Central compartment drug concentration, c X :
Where the volume of distribution, VC, was calculated via Eq. 3 as discussed in the main text.
Peripheral compartment drug mass, m P :
Equation B - 4 is subject to the initial condition mP = 0 at time t = 0
ith tracheobronchial airway compartment drug mass, mi (0 ≤ i < 14):
ith tracheobronchial airway compartment drug mass, mi (i = 14):
ith tracheobronchial airway compartment drug concentration, ci (0 ≤ i ≤ 14):
Alveolar compartment drug mass, m ALV :
Equation B – 5, B – 7, and B – 10 are subject to the initial condition mi,1 = Fi at time t = 0. Eq. B – 6, B – 8, and B – 11 are subject to the initial condition mi,2 = 0 at time t = 0
Rights and permissions
About this article
Cite this article
Ruzycki, C.A., Murphy, B., Nathoo, H. et al. Combined in Vitro-in Silico Approach to Predict Deposition and Pharmacokinetics of Budesonide Dry Powder Inhalers. Pharm Res 37, 209 (2020). https://doi.org/10.1007/s11095-020-02924-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02924-7